
Current guidance for absorption of elemental impurities does not address dermal exposure, resulting in a simplistic approach to limit setting.

Current guidance for absorption of elemental impurities does not address dermal exposure, resulting in a simplistic approach to limit setting.

Ensuring data integrity involves effort on an individual and global basis.

To enable efficient monitoring systems, life-science companies need to effectively apply run rules.

Mettler Toledo’s RX-10 Reactor Control automates jacketed lab reactors and controls thermostats, stirrers, and pumps.

Flow chemistry systems from Dolomite Microfluidics can be configured to suit different applications.

Shimadzu Scientific Instruments reports its EDX-7000/8000 spectrometers are compliant with electronic signature regulations.

The SFT - HPR-Micro Reactor from Supercritical Fluid Technologies introduces a is a pressure reactor for small batch reaction chemistry.

A high-pressure lab homogenizer from GEA for product development ensures efficiency and safety.

Kate Kuhrt, senior director, Generics and Biotech, Thomson Reuters, spoke with Pharmaceutical Technology Europe about sourcing trends, supply chain challenges, the emerging market outlook, and how it affects European pharmaceutical manufacturers.

Shimadzu’s newLCMS-8060 is designed to push the limits of LC/MS/MS quantitation for applications requiring the highest sensitivity and robustness while delivering a meaningful solution for routine LC/MS/MS analyses.

GEA's self-contained homogenizer is designed for laboratory applications, including cell dispersions.

A study of root cause in stability samples suggests the need for tighter control of the sodium lauryl sulfate manufacturing processes.

Shimadzu’s LCMS-8060 triple quadrupole mass spectrometer is designed for LC/MS/MS quantitation of highly sensitive applications.

After a long wait for the new elemental impurities guidelines, the bio/pharma industry must now look ahead to implementation and take action.

SafeBridge Consultants reports on the status of nine companies in the Potent Compound Safety Certification Program.

Shimadzu has introduced the i-series of integrated HPLC and UHPLC systems. The analyzers meet the needs of any analytical environment with high speed, outstanding performance, maintainability and economic efficiency.

The new sizes follow the 2014 launch of the company’s fully disposable purifier.

Charles Rivers strengthens its endotoxin testing and bacterial identification detection capabilities with the addition of Celsis’ products.
Biologics exhibit greater variability in stability testing than do small-molecule drugs, and maintaining a stable test environment is crucial.

Heightened regulatory scrutiny of data integrity highlights the need for comprehensive procedural reviews and strategies for managing mission-critical information.
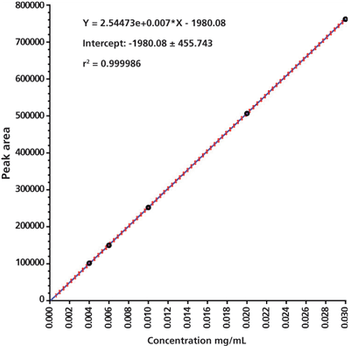
Statistical procedures give statistical answers not analytical judgement.

Thermo Scientific’s multimode microplate reader, the Varioskan LUX, is designed with intuitive software to allow for automatic and versatile functionality when performing microplate assays.

The new column features Natrix’s signature macroporous hydrogel.

BPTF seeks changes in performance goals and fee payment schedule in GDUFA renegotiations.

The Spectroscout portable X-ray fluorescence analyzer provides at-line elemental analysis.