Statistical analysis shows how much testing is needed to deliver a reliable estimate result.

Statistical analysis shows how much testing is needed to deliver a reliable estimate result.

The European Pharmacopoeia Commission re-evaluates its policy on the development of monographs for finished drug products.

Experimental work on wet granulation demonstrates the use of dynamic powder characterization to support continuous processing for tablet manufacturing.

Challenges encountered when implementing a continuous monitoring system are reviewed.

The effect of absorbed vapor-phase hydrogen peroxide on a lyophilized product Protein Z, was studied by spiking experiments with different amounts of hydrogen peroxide.

Industry experts discuss how extractables and leachables studies are designed using a risk-based approach.

Mass Spectrometer Enhances Performance in Protein Analysis

Data integrity in the analytical laboratory is an area of increasing focus for regulators such as FDA.

Aggregation System Improves Analysis of Sub-Visible Particles

Microscope Enables High-Resolution Materials Analysis

Laser Diffraction Software Supports Analytical QbD

A new process analytical technology based on impedance spectroscopy has potential applications for characterizing product attributes during the freeze-drying process.

7900 ICP-MS Increases Efficiency
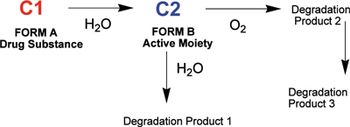
In this article, the authors demonstrate that a normal-phase chromatographic method was stability-indicating for a water-sensitive prodrug. The stress conditions using aqueous and non-aqueous conditions were also compared to understand the information obtained with each approach.

Compact Mass Spectrometer Improves Analysis

ACQUITY QDa Detector Increases Productivity

Advances in technology are improving the sensitivity and accuracy of mass spectrometry, increasing its use for the analysis of extractables and leachables.

Advanced triple quadrupole technology for improved performance and ease of use.
Automated sample handling, advanced glycan analysis, and specially designed columns are help speed up confirmation of the biosimilarity.

Simple and affordable mass detection for the chromatographer.

For a drug-development process that relies on outsourced services, special considerations are needed to ensure the proper transfer of technology and information from one phase to the next.
New simulation software improves modeling for chemists and materials scientists.

USP announced the approval of General Notices section 5.60.30 Elemental Impurities in USP Drug Products and Dietary Supplements with an official date of Dec. 1, 2015.
NMR analysis provides crucial structural information of synthesized glycans while LC-MS/MS is ideal for quantitation of free sugars in biological matrices.
Antibody fragments pose unique challenges in terms of recovery, purification, and formulation.