
Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.

The authors survey deficiency letters issued for 190 drug master files supporting abbreviated new drug application submissions.
This article introduces the concepts of pooled variance and the central limit theorem, which are intended for establishing acceptance criteria for blend uniformity data of granular powder blends when a significant degree of sampling bias is involved.
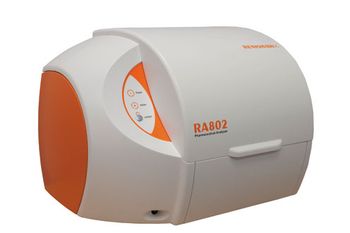
The RA802 Pharmaceutical Analyzer from Renishaw is a compact benchtop Raman imaging system that enables users to formulate tablets by speeding up the analysis of tablet composition and structure.

Analytical products for improved bio/pharmaceutical development.

Supply chain risk monitoring is crucial for any company doing business today, and it doesn’t have to be expensive.

PAT, quality by design, process controls, and analytical advances for small- and large-molecule drugs are on agenda for IFPAC 2017.

Aptar Pharma will showcase eDose Counter for MDIs integrating a proprietary sensing technology, at the Pharmapack Europe conference to be held in Paris, France on February 1-2, 2017

Camfil Air Pollution Control has expanded its testing laboratory and added a dust collection test rig for the ANSI dust collection standard.

Researchers were not able to replicate all of the findings in five highly-cited cancer biology papers.

EDQM outlines the steps it will take to implement the ICH Q3D guideline on elemental impurities.

B&W Tek’s director of Market and Customer Development, Katherine A. Bakeev, PhD, discusses analysis of pharmaceutical raw materials and the benefits of using handheld Raman spectrometers.

The European Pharmacopoeia Commission adopted a chapter on chemical imaging for analytical development, quality control, and manufacturing.

Analytical technologies play a key role in the characterization and quantitation of oligonucleotide therapeutics.

The authors present a set of statistical decision rules based on linear regression models that can be implemented in an automated trend system to assist stability studies.
Isothermal titration calorimetry and differential scanning calorimetry are valuable tools that can help accelerate drug development.

The integration of a method validation, transfer, and verification process into the overall lifecycle management process of a product can best align the variability of the analytical procedure with the requirements of the product.
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.

The Uniformity of Dosage Units test is used to evaluate content uniformity or weight variation or dosage forms such as tablets and capsules. The author introduces two different acceptance value limits (n = 10 and 30) in this article.

Agilent’s new facility in Folsom, California includes laboratory, order fulfilment, and warehousing space.

New software platforms aim to help manufacturers implement connected technology and use data efficiently.

The half-cycle method for validating sterilization can have adverse effects on materials if used for steam sterilization.

Dedicated facility will address enhanced regulation of metals and impurities in pharmaceuticals.

Idifarma has announced a growth of more than 50% over the past two years. Revenue is expected to hit approximately EUR5.3 million in 2016, growing from EUR3.5 million in 2014.

The article proposes an integration of method validation, transfer, and verification process into the overall lifecycle management process.