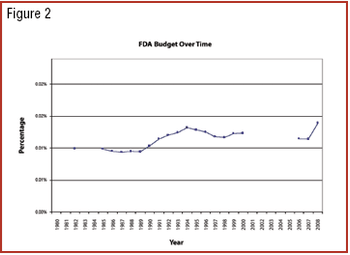
Can previous trends of Democratic and Republican administrations predict industry's future?

Can previous trends of Democratic and Republican administrations predict industry's future?

A book offers a detailed introduction to the new area of risk evaluation and mitigation strategies.

Cycle design and robustness testing using advanced process analytical technology.

A public meeting is being held today to discuss over-the-counter cough and cold medications for pediatric use.

Also, Alpharma advises shareholders to reject King's offer; ImClone rejects raised BMS offer; Immunogen appoints Daniel M. Junius, more...
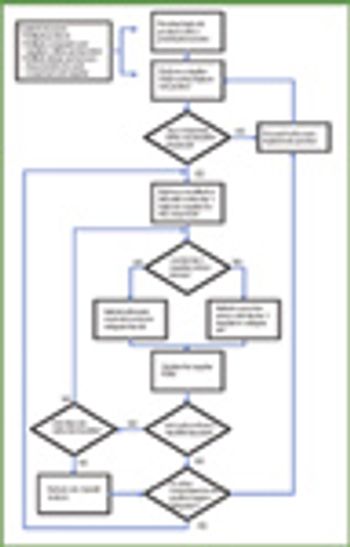
The growth and globalization of the pharmaceutical supply chain make risk assessment more important than ever for pharmaceutical manufacturers. The authors describe a program to identify, prioritize, mitigate, and communicate risks in manufacturer–supplier relationships.
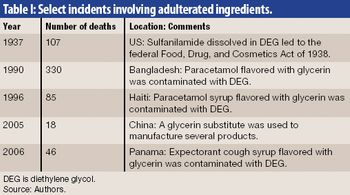
Regulatory bodies around the world are now revising legislation, regarding counterfeit medicines, good manufacturing and distribution practices, and risk management.

California's ePedigree requirements call for item-level serialization beginning in 2011. The author explains factors to consider when implementing a serialization strategy and how to achieve a positive return on investment.
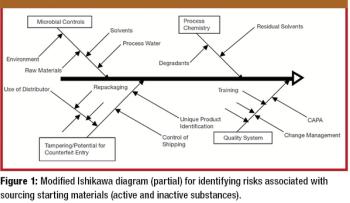
Securing the integrity of the excipient supply chain is a crucial task in ensuring the overall pharmaceutical supply chain. The authors outline excipient-control strategies and practices for the manufacture, distribution, and receipt of excipients.
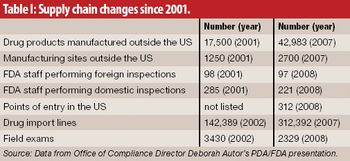
Pharmaceutical Technology has summarized recent statements by FDA officials on supply chain issues to provde the agency's most up-to-date views and expectations.

The design of accurate and robust analytical methodology is instrumental to developing orally inhaled and nasal drug products (OINDPs) and their appropriate control programmes.

The most public argument against direct-to-consumer advertising in Europe is essentially paternalistic: to protect people from companies who are considered unable to present an objective account of their drugs.

Also, Zentiva accepts Sanofi's increased takeover bid, Oriel Therapeutics appoints Richard Fuller CEO, more...

In a Sept. 17 letter to FDA Commissioner Andrew C. von Eschenbach, Rep. Henry Waxman (D-CA) questions the agency's priorities, specifically poking at FDA's political appointees and whether they are promoting industry at the expense of the public's health.

A new study concludes that an approval pathway for affordable follow-on biologics should be based on the Hatch–Waxman Act of 1984.

In an effort to clarify its policy on the use and creation of genetically engineered animals (GE animals), the US Food and Drug Administration released the draft guidance "The Regulation of Genetically Engineered Animals Containing Heritable rDNA Constructs" on September 18.

The US Food and Drug Administration has issued two warning letters to Ranbaxy Laboratories.

In November, representatives to the International Conference on Harmonization will meet in Brussels, Belgium, to discuss several international cooperation initiatives, including ICH Q10: Pharmaceutical Quality System and ICH Q8R: Pharmaceutical Development.

Earlier this month, the US Food and Drug Administration announced that it will be posting quarterly reports on its website regarding potential drug safety issues.

MannKind and Pfizer (New York) agreed that the former company will help patients who need inhaled insulin switch from Pfizer's "Exubera" medicine to MannKind's "Technosphere Insulin" drug.

The US Food and Drug Administration launched a new website to educate the general public about direct-to-consumer (DTC) advertising of prescription medications.

The US Food and Drug Administration has updated its draft bioequivalence recommendations for several products, and added 66 new draft product-specific guidelines since October 2007.

The European Chemical Industry Council and five national chemical associations representing France, Germany, Italy, Spain, and the United Kingdom, have launched ReachLink. ReachLink is a company founded to help companies participate in the Substance Information Exchange Forum (SIEF), which is designed as an information-sharing vehicle to facilitate companies in meeting requirements under REACH

FDA has issued a Final Rule titled "Amendments to the Current Good Manufacturing Practice Regulations for Finished Pharmaceuticals."

Also, Human Genome Sciences enters pact with Hospira, Zosano Pharma names Gail Schulze chair and CEO, more...