
The European Commission (EC) published a preliminary report stating that competition in the pharmaceutical industry does not work as well as it should.

The European Commission (EC) published a preliminary report stating that competition in the pharmaceutical industry does not work as well as it should.

When accusations fly: 'Tis better to give than to receive.

The past year saw major acquisitions attempted, completed, rejected, and stalled.

Readers provide insight into the best companies to work for as well as the ups and downs of their jobs.
While the world pulls itself out from one of the worst crises in decades, Indian pharmaceutical companies are trying to capitalize on falling company prices by increasing their takeovers.

To expand coverage amidst the economic crisis, Obama will look for ways to cut healthcare costs.

China's quality approach to domestic versus exported products seems to be a lose-lose situation.
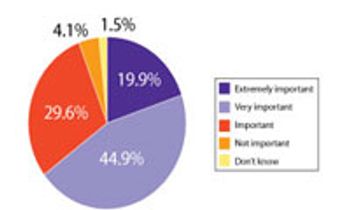
A recent Pharmaceutical Technology survey examined the level, sources, and reasons behind innovation in drug development and manufacturing. This article contains bonus online-exclusive material.
Pharmaceutical Technology is pleased to recognize the winners of its Innovations in Pharma Science Awards.
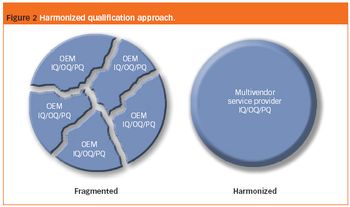
How has pharmaceutical manufacturing validation influenced analytical instrument qualification during the last 20 years and what are the emerging trends for the future?

The closing date for comments to be received by the US Pharmacopeia (USP) for proposed revisions to Chapter <231>, which deals with analysis of heavy metals, is 15 December 2008. The USP has been working towards for approximately 4 years and the task has not been easy.
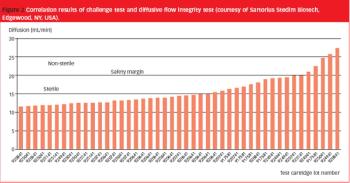
Although realized in 1983, ASTM's standrad test method for determining bacterial retention of membrane filters used for liquid filtration is still hugely beneficial to today's pharmaceutical industry.

...the new administration is not anti-science or even anti-industry. It just wants lower prices.

Also, Johnson & Johnson will acquire Omrix Biopharmaceuticals for $438 million; Charles River Laboratories promoted Foster Jordan to corporate senior VP of endotoxin and microbial detection products; more...

The US Food and Drug Administration issued a draft guidance, Contents of a Complete Submission for the Evaluation of Proprietary Names, on Nov. 24, 2008.

In a letter to Acting Comptroller General Gene Dodaro dated November 19, 2008, Congressman Joe Barton (R-Texas) requested that the US Government Accountability Office (GAO) review the US Food and Drug Administration's handling of the highly-publicized, tainted heparin scare that occurred in 2007 and 2008.

Ranbaxy Laboratories (Gurgaon, Haryana, India) responded to an investigation of violations at two of its manufacturing plants, according to a Reuters Health report.

Although the number of annual new-drug approvals in the United States has steadily declined during the past ten years, pharmaceutical companies still prefer to introduce new products in the US first, according to a report published by the Tufts Center for the Study of Drug Development.

The US Food and Drug issued a draft guidance on Tuesday, Nov. 18, titled Process Validation: General Principles and Practices for comment. The guidance is meant to serve as a revision to the 1987 Guideline on General Principles of Process Validation.

US marshals seized 11 lots of heparin from Celsus Laboratories (Cincinnati, OH) at the request of the US Food and Drug Administration.

Also, Patheon opens new European headquarters, Cynvec appoints Frank D. Stonebanks president and CEO, more...

The Steering Committee and Expert Working Groups of the International Conference on Harmonization are meeting this week in Brussels to discuss current harmonization efforts.

PAT may reduce costs by helping companies control process variability, improve yields, reduce waste, and produce high-quality therapies consistently. Companies that have not yet embraced PAT may find its potential to reduce expenses a compelling argument in its favor during this time of financial difficulty.

The European Medicines Agency's (EMEA) Committee for Medicinal Products for Human Use recommended that the status of GlaxoSmithKline's anti-obesity drug "Alli" (orlistat) be switched from prescription-only to nonprescription.

The US Food and Drug Administration sent Warning Letters to Bayer HealthCare (Morristown, NJ) about the company's "Bayer Women?s Low Dose Aspirin + Calcium" and "Bayer Aspirin with Heart Advantage" over-the-counter (OTC) products.