
FDA is taking several measures to ensure that imported drugs meet manufacturing standards.

FDA is taking several measures to ensure that imported drugs meet manufacturing standards.
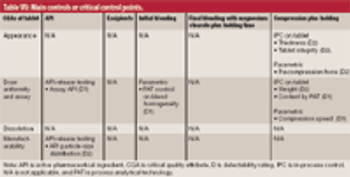
Criticality management combines pharmaceutical product, process, and material knowledge and risk management in one approach, which is reflected in a single document.

Operators are hit hard by breakage problems and strike out on FDA inspections.

Are hypersanitation trends a result of scaremongering or a lack of faith in medicine?
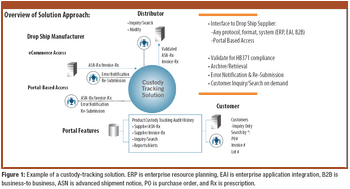
EPedigree, track-and-trace technologies, and other tools for optimizing supply-chain management are of increasing importance to the pharmaceutical industry. The author examines the current regulatory and legislative framework for ePedigree for finished drug products as well as proposals to require electronic statements for pharmaceutical ingredients.
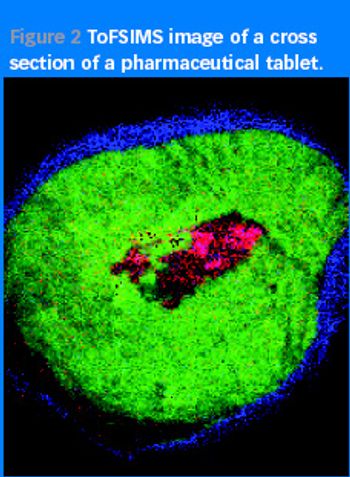
As counterfeiters become more cunning and technologically advanced, spotting their handiwork is increasingly difficult. Can surface analysis techniques be used to outwit them?

The authors describe the critical aspects of an ideal fermentation services provider.

The US Food and Drug Administration will hold a public hearing Oct. 2, 2008, to obtain input regarding over-the-counter (OTC) cough and cold drugs marketed for pediatric use.

FDA has finalized a regulation regarding changes to an approved new drug application (NDA), biologics license application (BLA), or medical device premarket approval applicatin (PMA).

The US Pharmacopeial Conventional added to its international sites with the official opening of its facility in Sao Paulo, Brazil.

The Synthetic Organic Chemical Manufacturers Association (SOCMA) is expressing trade concerns with the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, a new European Union policy on chemicals and their safe use.

The concept of the the triple bottom line incorporates a company's financial, social, and environmental performance. As a result, an increasing number of companies are investigating how sustainable, green technologies and practices can help them stay competitive in a challenging regulated market.

Decontaminating pharmaceutical manufacturing facilities is essential for producing safe and pure drugs. The most commonly used decontaminant in the industry has been sublimated formaldehyde.

The US Food and Drug Administration issued a new draft guidance for public comment on sterile manufacturing.

Actavis Totowa LLC, the US subsidiary of the generic drug manufacturer Actavis Group, is announcing a voluntary recall to the retail level of all drug products manufactured at its Little Falls, New Jersey, facility. This is a precautionary, voluntary action by Actavis following an inspection conducted by the Food and Drug Administration earlier this year.

The US Food and Drug Administration has distributed a draft of Guidance for Industry: Residual Solvents in Drug Products Marketed in the United States for public comment.

The US Food and Drug Administration seeks contractors that will identify, describe, and evaluate potential data sources or data environments that could participate in the agency's Sentinel Initiative.

Also, Ortho Biotech recalls one lot of "Procrit" due to cracked vials, more appointments at Actavis, more...

The US Food and Drug Administration released its user fee rates for fiscal year 2009 last Friday.

The pharmaceutical industry?s outsourcing practices receive increased attention as Congress evaluates the role of outsourcing in drug-safety issues.

Agents report unusual chemistry, abnormal data analysis, and unconventional work practices.

Myth versus Reality: What does Q10 implementation really mean for my company?

Surrounded by competition, Vietnam's 2020 vision focuses on building a biotech sector worthy of its Asian neighbors-as well as the growing global biopharmaceutical market

Legislative decisions to increase Medicare's formulary may lead to a fight over drug approvals.

USP's guideline for pending monographs can speed up publication of monograhs and time to market.