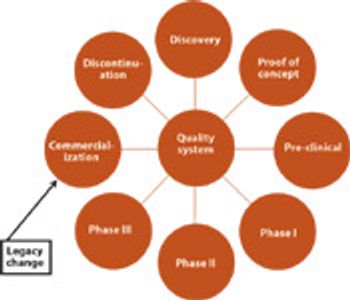
The concept of the the triple bottom line incorporates a company's financial, social, and environmental performance. As a result, an increasing number of companies are investigating how sustainable, green technologies and practices can help them stay competitive in a challenging regulated market.