Wyatt Earp, the legendary sheriff of Tombstone used to solve troubles in a simple way: aiming, pulling the trigger and bang! ... problem eliminated! Some manufacturers check the readability of their barcodes in the same way.

Wyatt Earp, the legendary sheriff of Tombstone used to solve troubles in a simple way: aiming, pulling the trigger and bang! ... problem eliminated! Some manufacturers check the readability of their barcodes in the same way.

To date, the pharmaceutical industry?s primary defence to counterfeiting is to create more sophisticated packaging that makes imitation more difficult, such as packaging involving barcoding and RFID.

Company and People Notes: Valeant and Biovail to Merge; GPhA Names Interim Director; And More.

European pharmaceutical companies are outdoing their US counterparts when it comes to making medicines available to developing countries, according to the Access to Medicine Index, which analyzes and ranks the access to medicine efforts of the world's largest pharma companies.

GSK Acquires Laboratorios Phoenix; Catalent Makes Executive Appointments; And More.

Thermo Fisher to Acquire Fermentas; Pfizer Names Head of R&D; And More.

The US Food and Drug Administration began a partnership with the website Drugs.com to expand access to the agency's consumer-health information.

About one month after the announcement of McNeil Consumer Healthcare's recall of children's liquid pain and allergy medications , the US Food and Drug Administration testified before the US House Committee on Oversight and Government Reform about the issue.

USP membership meeting prepared the standards-setting body to meet modern challenges.

Editors' picks of pharmaceutical science and technology innovations.

A timely new book explains techniques for conformational analysis.

Europe moves to place excipient GMP and GDP standards on the same level as active pharmaceutical ingredients.

Too much or too little control can actually lead to the same result.
The authors recommend a strategy for classifying similar nonstainless-steel surfaces into three groups based upon the analytical recovery that was observed in this study.

Granulation is the most crucial step in the manufacture of solid dosage forms.

The requirements of a granule drying process haven't really changed much in recent years.

A laboratory information system (LIMS) is a key tool in facilitating communication between a pharmaceutical company and its outsourcing partners; however, most LIMS require extensive customisation before they can be used in the pharmaceutical industry.

An effective quality management system will identify the root cause of non-conformances and put measures in place to ensure that they do not recur, but is the pharmaceutical industry choosing the right methods to make sure this happens?

An environmental risk assessment (ERA) is now required for all new pharmaceutical product marketing authorisation applications. The author outlines the steps to this procedure.

Compressed tablets are the most popular solid dosage form and, despite having been used for many years, still offer numerous advantages.

The market for tablet compression technology and the demands placed on equipment manufacturers have changed quite significantly in recent years.

Shared audits of suppliers offer several advantages, but networking to conduct such an audit can be challenging.

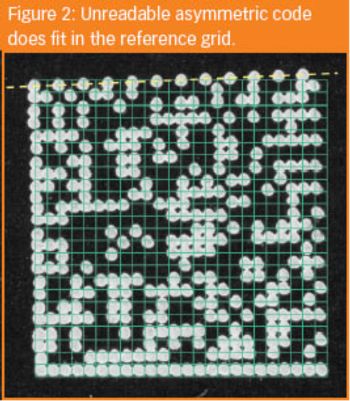
Tablet coding or identification is based either on the detection system being overt (visual to the naked eye) or covert (non-visual). The coding of tablets is obvious at an overt level because of size, shape, colour and logo design.

We regularly deal with tabletting equipment problems and malfunctions, and find that the most common cause is misuse or improper handling and transportation, which often leads to accidental damage to tablet punches (including nicks and dents to the punch tip edges).

Despite its potential, why is personalised medicine still not widely used in healthcare?