
Many pharmaceutical companies in the UK have adopted a direct-to-pharmacy distribution model, which enables companies to more tightly control their supply chains.

Many pharmaceutical companies in the UK have adopted a direct-to-pharmacy distribution model, which enables companies to more tightly control their supply chains.

The health of the contract-services industry is improving but the market is signaling tougher competition ahead. This article is part of the 2010 Outsourcing Resources special issue.

Overall Equipment Effectiveness has been shown to identify the root cause of inefficiencies.

Representatives of SOCMA and IPEC explain new quality agreement templates and their use for meeting regulatory expectations and securing the pharmaceutical supply chain.

A new guideline for conducting bioequivalence studies was adopted by the CPMP in January and it becomes fully effective as of August 2010.

The authors describe recent market trends and indicate the likely future direction of the pharmaceutical and biopharmaceutical industries. This article is part of the 2010 Outsourcing Resources special issue.

We speak to The European generic Medicines Association about the environment for biosimilars.

The recent amendment to Annex 1 has seen controversial changes relating to the capping of vials.

New isolator and disposable technologies are set to assume a greater role in pharma manufacturing, according to a recent conference in Germany.

The EMA's Guideline on Similar Biological Medical Products provides overarching guidance, but since its introduction a range of more specific guidelines have also been developed.

As biopharmaceuticals will soon make up 50% of new drug approvals there has been a significant rise in interest in the field of biosimilars.

The biggest differences between the development of a biosimilar and a generic medicine lie primarily in the preclinical and clinical stages.

The author describes the importance of risk management and offers strategies for risk-management preparedness in supply chains.

Why the primary defence against biosimilars is to develop better molecules that can be launched quickly.

With more hands in the manufacturing pot, a good contingency plan is crucial to success.
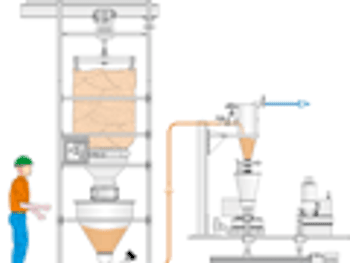
What are the newest trends in pneumatic conveying of pharmaceutical powders and blends?

Leading CMOs share their experiences with regard to client expectations and performance indicators as well as strategies for adapting to the changing contract-manufacturing environment. This article is part of the 2010 Outsourcing Resources special issue.

Merck and Sinopharm Sign Agreement; Agilent Appoints CFO; And More.

In 2009, the European Commission (EC) Customs Union seized 11,462,533 medicines and medical products for suspected violations of intellectual property (IP), according to the Commission's annual report on EU Customs Enforcement of Intellectual Property Rights, which was published last week.

Researchers claim that a dissolving microneedle patch may be able to offer improved vaccination against influenza compared with traditional needles, and also allow people without medical training to easily and safely administer the vaccine.

Pfizer Ends Second Tanezumab Clinical Program; Catalent VP Joins USP Panel; And More.

The Society of Chemical Manufacturers and Affiliates (SOCMA) expressed "strong concern" over legislation introduced in the US Senate earlier this month regarding chemical-site security.

Merck KGaA (Darmstadt, Germany), a global pharmaceutical and chemical company, completed its acquisition of Millipore (Billerica, MA), a life-science company, last Thursday for an aggregate purchase price of roughly EUR 5.2 billion ($6.7 billion).

Teva's and Sun Pharmaceuticals' motion to overturn a verdict issued against them in April 2010 for patent infringement has been denied by a US court, leaving the companies at the mercy of pharma giants Pfizer and Nycomed, who have said they will "vigorously" pursue damage claims.

The pharmaceutical industry?s increasing interest in inhaled drugs has prompted several researchers to propose standard dissolution-testing methods for these products.