
A look at why Brazil revised its GMP standards and how the changes will affect the local pharmaceutical industry.

A look at why Brazil revised its GMP standards and how the changes will affect the local pharmaceutical industry.

Pending legislation may give FTC the authority to regulate all Hatch-Waxman settlements.

Pharmaceutical Technology gains insight into approaches for producing aromatic amines.

From weekend deliveries to nonsterile gloves, a single exception can make a product fall flat.

Software and online monitoring are helping the pharmaceutical industry improve its corrective and preventive action programs. This article contains bonus online material.

PharmTech talked to anticounterfeiting experts to discuss terrorism as a source of counterfeit products.

Industry participation is crucial as USP embarks on far-reaching monograph modernization initiative. This is an online-exclusive article.

New data provide insight into pharma-industry professionals' daily lives.
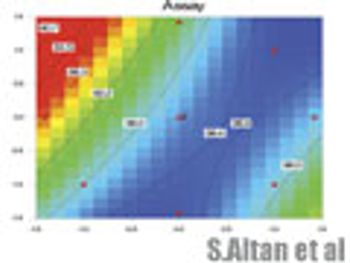
In the final article of a three-part series, the authors discuss how to present a design space and evaluate its graphical representation.

The President's Council on Advisors on Science and Technology (PCAST), a group administered by the federal Office of Science and Technology Policy (OSTP), issued recommendations to improve the country's ability to accelerate vaccine production and delivery in the event of a pandemic flu outbreak.

Genzyme (Cambridge, MA) confirmed that it had received sanofi-aventis's (Paris) proposal to acquire all of its outstanding shares for $69 each.

A Strengths, Weaknesses, Opportunities and Threats analysis of the biosimilars market is given.

Both biosimilar and generic drugs have an abbreviated approval process; however, the clinical trial requirements differ enormously.

Despite the poor economic climate, large-scale mergers and acquisitions in the pharmaceutical industry struck back with a vengeance in 2009.
Bristol-Myers Squibb's fit-for-purpose mode for clinical-trial materials for early-stage development seeks to achieve a better way in resource allocation. This article is part of a special issue on API Development, Formulation, Synthesis and Manufacturing.

The current trend in the pharmaceutical industry for the manufacture of small-molecule therapeutic agents is moving toward continuous flow processes. This article is part of a special issue on APIs.

In an increasingly competitive landscape, outsourcing providers are under mounting pressure to get their name out there and secure new and repeat business.

The author presents recent developments in simulated moving-bed chromatography in production of active pharmaceutical ingredients and intermediates. This article is part of a special issue on APIs.
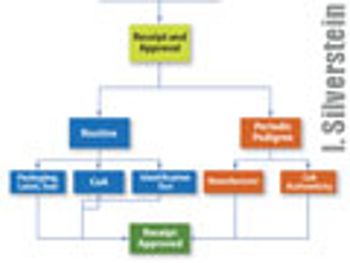
The author explains the background behind the excipient pedigree and how to implement its use.

Every biopharmaceutical is unique and products are defined as much by their manufacturing process as their analytical characterisation. Because of this, the development of biologics has many inherent complexities over small molecule projects.

The authors discuss a continuous-flow reactor that avoids parallel channels and enables economic plant setup. This article is part of a special issue on API Development, Formulation, Synthesis and Manufacturing.

In light of the impressive size and predicted growth of the market, there has been a rising interest in the development of biosimilars.

The manufacture of high potency active pharmaceutical ingredients (HPAPIs) is on the rise with R&D projects showing a continuing interest in these products.

Industry experts discuss the merits of continuous processing technology and explain why pharma manufacturers should realize the benefits these technologies offer.
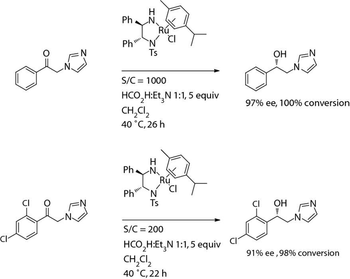
The authors describe several examples of using asymmetric hydrogenation and biocatalysis for synthesizing several secondary alcohol compounds.