
Company and People Notes: Novartis Settles with US Attorney's Office; Hospira Names VP, And More.

Company and People Notes: Novartis Settles with US Attorney's Office; Hospira Names VP, And More.

The reopened debate over embryonic-stem-cell research could stifle many other scientific pursuits.

Private companies and universities are developing new ways to deliver protein drugs.

Public-private R&D partnerships are on the rise across Europe, but national goals and academia-industry competition could prevent their success at the European level.

Global pharmaceutical companies could have a problem getting rid of redundant facilities.

Editors' picks of pharmaceutical science and technology innovations.

An expert-panel-written book has surprising shortcomings.
Drugmakers hatch new manufacturing paradigms in the wake of the 2009 H1N1 influenza pandemic.

From disagreement to denial, being cordial about quality control can be challenging.

Pfizer's acquisition of Wyeth involves augmenting its biopharmaceutical product portfolio, pipeline, and related development and manufacturing capabilities.

The president of Gibraltar Laboratories, Daniel Prince, discusses industry trends and challenges.

A look at MVI's malaria work in developing countries.

Novartis' Matthew Stober discusses vaccine manufacturing, including egg- and cell-based systems.

In light of compendial changes, representatives of the US Pharmacopeia and an industry consortium provide perspectives on cap and ferrule labels.
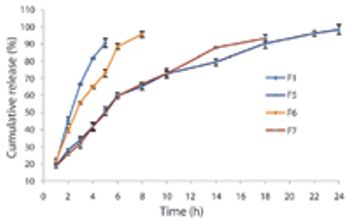
The authors developed a formulation for effervescent gastroretentive drug delivery techniques using ibuprofen as a model drug. They optimized the formulations by applying full factorial design.

President Obama and HHS eye innovation and countermeasures to protect public health.

There are a number of validation approaches that can be adopted for single-use systems - all of which incorporate an established approach.

Since its introduction more than 10 years ago, single-use bioreactor technology has now become an established addition to today's biotechnology manufacturing facility.

Single?use solutions, such as bags, tank replacements and aseptic connection devices (ACDs) have revolutionised our industry, providing improved sterility assurance.
There are apparent inconsistencies between the use of these reference materials and the manufacture of the reference solutions that are actually used in the regulated test method.

Life science industry users of single?use devices have legitimate questions about the potential for leachables to impact the quality of their pharmaceutical products.

There are several key advantages that single-use systems offer over conventional stainless steel equipment.

The willingness of the industry to use single-use bioreactors is currently influenced by the criticality of the step, the value of the product and the time for product development and production.

The early part of the decade saw a decline in vaccine sales and manufacture, but finally the industry is bouncing back, with Europe particularly well placed to make an impact.

The development of quality agreements has long been recognised as a critical activity to ensure a product's quality meets regulatory requirements.