
The challenge with any of the emerging markets is in adjusting to the western regulatory concepts of quality and compliance.

The challenge with any of the emerging markets is in adjusting to the western regulatory concepts of quality and compliance.

An Industry Roundtable Moderated by Patricia Van Arnum and Rich Whitworh. Contract service and technology providers share their perspectives on the influence of quality by design in the expectations between sponsor companies and outsourcing providers.

The European Medicines Agency has updated the template for package leaflets to make the information easier for patients to understand.

The global production of seasonal influenza vaccine will double to 1.7 billion doses by 2015, according to a World Health Organization presentation.

The DHS announced it has revised tiering assignments for several chemical facilities covered under DHS's Chemical Facility Anti-Terrorism Standards program, which requires chemical companies to develop and implement specific security plans for their facilities.

FDA Approves the Influenza Vaccine Formulation for the 2011-2012 Flu Season.

The pharmaceutical industry and US regulatory bodies have not responded adequately to the increasing level of outsourced manufacturing in countries such as China and India, according to a new white paper by the PEW Health Group.

Even though manufacturers are responsible for ensuring the quality of combination products, some companies may not be certain about what quality system to apply to their production.
![Rivera-Fig-4-new[2]web-731308-1408618780125.jpg](https://cdn.sanity.io/images/0vv8moc6/pharmtech/c5b3bd34e31edbde4e6a56be25a83c3d7a998f32-504x275.jpg?w=350&fit=crop&auto=format)
The author describes the components that make up a clean-in-place system and how the system should be built to ensure efficiency.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the July 2011 edition from BETE Fog Nozzle and Continental Disc.

We?re attempting to validate a clean-in-place process using total organic carbon (TOC) as the analytical method. Our swab-sample recovery tests are giving us inconsistent results. Recovery values range from 95% to 205%, and there seems to be no correlation with the amount of drug product deposited on the coupon. What could be the problem?
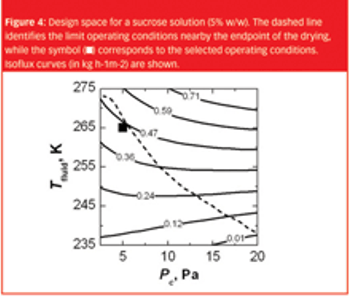
The same recipe obtained in laboratory-scale equipment cannot, without modifications, generally be used to freeze-dry the product in a pilot-scale or industrial-scale freeze-dryer.
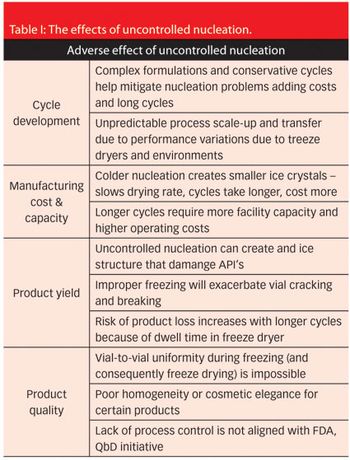
Ideally, all product formulations should experience the same conditions when it comes to lyophilisation, but in practice, however, nucleation can occur.

SOCMA has issued its support this week of the passage of pending free-trade agreements with South Korea, Panama, and Colombia by two Congressional committees.

FDA banned the importation of products manufactured at the Mexican unit of Dr. Reddy's Laboratories. The import ban is a result of the company's failure to correct the violations listed in a recent Warning Letter to the agency's satisfaction.

With a new North American headquarters and expanded operations, Almac is furthering its transatlantic approach to contract development and manufacturing.

A recent industry survey shows keen interest in improving bioreactors and cell-culture media.

The increase in aseptic processing driven by the growing number of biologically-derived products has led to an increase in freeze-drying applications in this area at both research and production scales.

A PQRI expert working group provides case study examples of risk-management applications.

The performance of biotechnology venture capital and investment is lackluster at best.

Getting an answer is easy-asking the right question is apparently more difficult.

Editor's picks of new manufacturing products for July 2011.

A new report places pharmaceutical and healthcare companies ahead in corporate and social governance.

The solid form of an API plays a crucial role in drug quality, and advancing methods for screening, detection, and characterization is key.

Member states in the EU are working to implement the newly passed Falsified Medicines Directive.