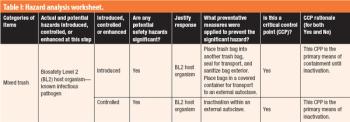
The third in a series of eight case studies from the Product Quality Research Institute focuses on facility biocontainment and inactivation.

The third in a series of eight case studies from the Product Quality Research Institute focuses on facility biocontainment and inactivation.

Biocatalysis, chemocatalysis, and other chiral technologies continue to attract the investment dollars of CMOs and fine-chemical companies.

The rising cost of healthcare has complicated the introduction of new, innovative drugs and other medical technologies. While the medical impact of these healthcare advances cannot be denied, governments and healthcare policy makers have found it difficult to sustain the funding of these expensive treatments.

Elham Blouet from Roquette explains the importance of carbohydrates for injection and the challenges in this niche market.
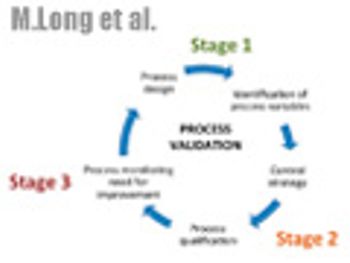
The authors desribe the three-stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation.

As contract manufacturers and fine-chemical suppliers gather for CPhI/ICSE, effective strategies for technology differentiation are key in an increasingly competitive environment.

By introducing a Patent Box scheme that offers reduced taxes to innovative companies, the UK government hopes to encourage investment in the country.

A Q&A with Joe Cascone, director of potent pharmaceutical development at Metrics, moderated by Patricia Van Arnum. Discussion of the key considerations made in facility design, equipment selection, and operations to achieve desired levels of containment.

Highly potent APIs (HPAPIs) represent a growing area of interest for the pharma industry. Mark Griffiths, CEO of Carbogen Amcis AG, explains why.

Henrik Schwartzbach, senior process technologist at GEA Niro, explins why spray drying is seeing increased uptake in the pharma industry.

A Q&A with Brian Johnson, senior director of supply chain security at Pfizer, moderated by Patricia Van Arnum. Part of a special Ingredients issue.
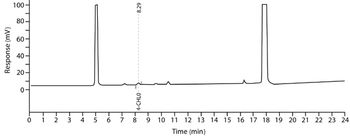
The authors provide an overview of methods for the quantitative determination of genotoxic impurities (GTIs) in active pharmaceutical ingredients.

Recently, there have been many innovations in the latest techniques and technologies in API purification. In particular, the trend to single-use systems has had a significant impact on processes.

Strategies to minimise particle levels in the finished product when using single-use technologies downstream of final filters.
A perspective from Pfizer on the lessons from small-molecule manufacturing that can be applied to biomanufacturing.

To further enhance its position in clinical-trial materials supply, Catalent Pharma Solutions has agreed to acquire the clinical-trial supplies business of Aptuit for $410 million on a cash and debt-fee basis.

Last week, Ben Venue Laboratories decided to exit the contract-manufacturing business during the next several years, thus ending more than 70 years of service in this field. To ensure the supply of medically necessary products, the company will work with its customers to develop and execute long-term transition plans.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2011 edition from Chemineer and IDS.

We try to use previous experience to develop the optimal granulation process for each new ingredient, but we sometimes get it wrong. Many times, we?ve taken materials from the granulator only to find that they exhibit poor flow and insufficient density. How can we easily determine the end point of a given granulation process?

Achieving a consistent level of quality control could greatly reduce waste and save money for the pharmaceutical industry. But why has talk about Six Sigma died down at a time when it could be of great benefit?
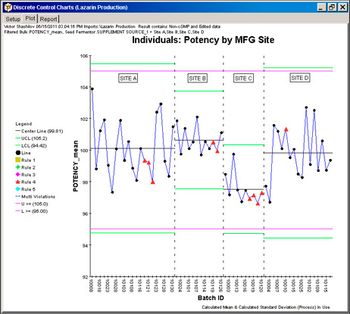
Using information technology tools to enhance process understanding helps reduce variability that can affect speed to market.

The Society for Chemical Manufacturers & Affiliates commented on EPA's modifications to the Inventory Update Reporting rule, also broadly know as the Chemical Data Reporting rule. EPA issued the final rule earlier this month.

Since the passage of the BPCI Act in 2009, manufacturers have been waiting for guidance from FDA on what that approval process will look like.

Xevo TQD provides ultimate consistency between analyses; instrument to instrument, lab to lab; both the advanced technology of the ACQUITY UPLC System and the robust universal ion source architecture on the Xevo TQD guarantee flexibility and dependability.

Merck & Co. announced last week that it plans to reduce its global workforce, as measured by year-end 2009 levels, by an additional 12–13% by 2015. The company made the announcement as part of its second-quarter earnings release, which the company issued on July 29, 2011.