
To find out how well industry is applying QbD, and what benefits the approach can bring, Equipment and Processing Report talked to Moheb M. Nasr, director of FDA?s Office of New Drug Quality Assessment.

To find out how well industry is applying QbD, and what benefits the approach can bring, Equipment and Processing Report talked to Moheb M. Nasr, director of FDA?s Office of New Drug Quality Assessment.

FDA recently published guidance for preventing the cross-contamination of finished pharmaceuticals and active pharmaceutical ingredients with nonpenicillin beta-lactam antibiotics.

Merck & Co. has formed a joint venture with the Mumbai-based specialty pharmaceutical company Sun Pharmaceutical Industries to develop, manufacture, and commercialize new combinations and formulations of branded generic drugs in emerging markets.

Johnson & Johnson has instituted a new structure for its Consumer Group according to a Reuters report.


After completing a strategic review of options for its Capsugel business, Pfizer announced on Apr. 04, 2011 that Kohlberg Kravis Roberts & Co L.P will acquire the pharmaceutical and dietary capsule company.

The Society of Chemical Manufacturers and Affiliates criticized the Secure Chemical Facilities Act, which would require chemical facilities to use inherently safer technology as part of chemical-site security measures.

Eli Lilly and GlaxoSmithKline are among several pharmaceutical companies using technology transfer as way to improve health outcomes in the developing world.

Chemspec USA, which will be held in early May, in Philadelphia, addresses the key issues affecting the manufacturing and supply of fine chemicals.

Editor's picks of analytical instrumentation products for April 2011.

Courts and Congress seek to reshape policies and programs.

An uncertain regulatory environment affects funding for biotechnology.

Research and development may be headed for divorce.

The contract-research industry in China is growing in leaps and bounds, and Big Pharma is leading the way.

FDA reviewers aim to assist ANDA sponsors in building quality into their submissions by clarifying components of the applications. Part 4 addresses manufacture and container closure.

Regulators question whether particles that they can't see hurt patients.

PhRMA efforts of industry's R&D scientists.

The authors revisit their previous effort to refine the terms that describe interventions and to dispel confusion that arose after the original article was published.

The complexity of third-party external supply networks requires new ways to manage them.

In any industry, inspections can be a pain, and pharma is no exception.

Companies engaged in global mergers and acquisitions may be hearing from the Department of Justice more often to ensure that corruptive practices are not taking place.
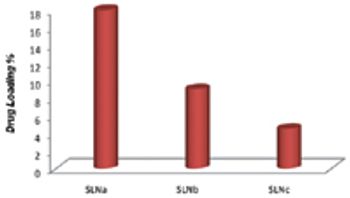
The aim of this study was to prepare and characterize physiochemically and biologically tamoxifen-loaded SLNs to evaluate their effectiveness as a drug-delivery system to treat breast cancers.

The need for greater process understanding raises the bar for suppliers.

Bob Weaver, president of HunterLab, discusses current trends and challenges.

This technical forum is part of a special issue on Solid Dosage and Excipients.