
Representatives of contract service organizations that develop biologic-based drugs discussed technology trends such as high-throughput screening and single-use systems.

Representatives of contract service organizations that develop biologic-based drugs discussed technology trends such as high-throughput screening and single-use systems.

Contract services ride high as funding floods bio/pharma.

Representatives of contract service organizations that specialize in parenteral drug development and manufacturing describe the evolving trends.

Despite the growth of specialist companies with capabilities across various therapeutic areas in Europe, there is still a need for early development expertise with end-to-end pharmaceutical manufacturing capabilities.

Contract development and manufacturing organizations identify trends, challenges, and emerging technology and service needs for solid and semi-solid dosage forms.

For a bio/pharma industry in flux, contract services are playing a greater-and more diverse-role in drug development.

Pfizer announced an agreement to acquire Baxter International?s marketed vaccines for $635 million.

The regulatory filing marks the first step in the approval process toward making RTS,S available as an addition to existing tools currently recommended for malaria prevention.

Packaging Coordinators, Inc. expands its services with acquisition of Penn Pharmaceutical Services.

Study provides first substantive reference data on key quality attributes of empty capsules

Sandoz is first to file for FDA approval of a biologic under the biosimilars pathway created in the Biologics Price Competition and Innovation Act of 2009.

American Health Packaging announced a voluntary nationwide recall of ibuprofen and oxcarbazepine tablets due to mislabeling on the inner unit dose blister packaging.

Unique Pharmaceuticals has issued a voluntary recall of sterile compounded preparations, but aired concerns about FDA?s recall demand.

Modular design and quick-changeover features help deliver flexibility and maximize return on investment.

Hovione will use Merrion's GIPET absorption-enhancing technology for solid-dosage drugs.

Isolators should be designed to enhance working conditions for end-users.
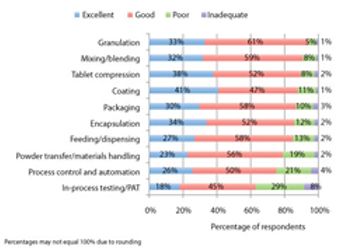
Pharmaceutical Technology’s survey collected industry feedback on trends and the utility of equipment used in finished drug-product manufacturing.

West's new plant in India will manufacture primary packaging for injectable drugs.

Puncture in container and overwrap cause leak in intravenous solution, leading to recall by Hospira.

Baxter Healthcare has initiated a nationwide recall of more than 20,000 containers of a pre-mixed beta blocker due to the presence of particulate matter.

The MG2 Selekta is a high-speed checkweigher and sorting machine for tablet production.

Fette's FE75 Tablet Press is designed for large batches.

A hygiene-grade conveyor chain from FB Chain improves wear compared to stainless-steel chains.

Gerresheimer's Gx G-Fix adapter integrates prefilled glass syringes with polymer-based autoinjector devices.

Kraemer KD60 equipment conveys and dedusts vitamin and nutraceutical tablets.