
Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the benefits of automated processes.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the benefits of automated processes.
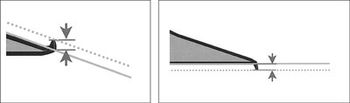
To achieve the high quality standards required for critical defects in pharmaceutical glass syringes, a combination of visual and camera-based inspection technologies are used.

InnoPack, which runs alongside CPhI Worldwide in Paris Nord Villepinte, France, is specially focused on pharmaceutical packaging. This is the place where companies gather to showcase their latest innovations and technologies in packaging. In this article, Martin Dallas from Essentra explains the role of innovative packaging technologies.

NIH seeks new therapeutic products to bolster the protective properties of vaccines.

Multidirectional collaboration is critical for the new pharma business model; cloud-based information services can offer a communications alternative.

Partnership is awarded for licensing of low-cost vaccine for the treatment of bacterial meningitis.

Meeting increasing expectations and escalating regulatory requirements to protect patients.

I Holland, in collaboration with the University of Nottingham and experts from the Laboratory of Biophysics and Surface Analysis, United Kingdom, recently completed a two-year tableting science anti-stick research (TSAR) project, which sought to understand why certain formulations stick to tablet tooling.

Leading global tablet tooling manufacturer, I Holland has a range of multiple tip punches and dies optimised to meet the requirements of modern tablet production.

CALIXAR and VirPath use new manufacturing techniques to create a vaccine for the pandemic flu, influenza A (H1N1), available in 2015.

Regulations, product protection, and cost management are top concerns of supply chain decision makers.

GS1 publishes a healthcare industry guideline describing how to implement GS1 standards to support requirements of the 2013 US Drug Supply Chain Security Act.

Texas A&M dedicates national pandemic influenza vaccine manufacturing facility.

GPhA throws its support behind a bill to prohibit companies from using REMS practices to deter competition.

Micronization and other processes are used to obtain optimal particle characteristics for pulmonary and oral solid-dosage delivery.

The EdgeTRAC software Print, Apply, and Verify module from ROC IT Solutions can be used to implement serialization in manual packing operations.

Understanding how to identify, remediate, and prevent facility infection is crucial for product quality.

A semi-automatic case packer from Omega Design and Brazil's Grupo Tecnor offers unit-level serialization capabilities.

Designed for ISO Class 4 compatibility, BioClean? 400mm (16?) gloves give 33% more coverage than the standard cleanroom glove providing exceptional comfort and offering extra personal and product protection.

Molecular Profiles has expanded its capsule filling capability following significant investment into new equipment at its clinical manufacturing site in the United Kingdom.

Kurt Lumsden, Director, eCDS Client Services at PAREXEL Informatics, discusses the use of interactive response technologies in investigational product expiry management.

FDA approved XTANDI for the treatment of metastatic castration-resistant prostate cancer.

The pharmaceutical industry has traditionally used glass as a primary material for containment systems due to a variety of characteristics that enable generally safe and efficient drug storage. However, there are many risks associated with glass.

Terra Universal introduced a floor-mount model of its BioSafe pass-through for cleanroom material transfer.

FDA gives NewLink Genetics approval to proceed to Phase 1 clinical studies of Ebola vaccine.