
Biotage has launched a purification technology, Accelerated Chromatographic Isolation (ACI), which converts simple flash purification into a faster and more economical way to isolate pure compounds.

Biotage has launched a purification technology, Accelerated Chromatographic Isolation (ACI), which converts simple flash purification into a faster and more economical way to isolate pure compounds.

PharmSource special report shows demand for cytotoxic injectable drugs could tap existing CMO capacity.

Aesica Pharmaceuticals S.r.I. collaborates with QAD to meet China&s Food and Drug Administration's shortened serialization deadline.

New services manage clinical or drug manufacturing data in the Verizon cloud or from data centers.

Arecor and the Center for Process Innovation (CPI) Biologics are working on a project to enhance the compatibility of biologics with their containers.

New advanced aseptic manufacturing technologies are available for filling liquid pharmaceuticals, including biologics.

Funding boosts life-sciences manufacturing in West Michigan.

The Flexi-Cap features a first-opening indication as an anticounterfeiting solution.

Novartis facility becomes the first US site licensed by the FDA to produce cell-culture influenza vaccines.

The fabrication facility will supply modular construction to the US life-science market.

A new report from GlobalData states that biosimilars will overtake the market share after 2019.

The vacuum laboratory filtration and drying unit is available in metal for higher pressures.

Innovative polyethylene film sets new benchmark
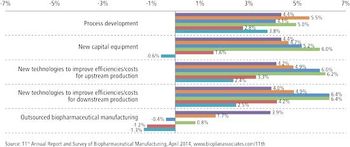
With budgets growing, clients see CMOs' costs as less crucial.

New packaging concepts and equipment, including systems for vial handling, printing and labeling, and parenteral packaging, were revealed at INTERPHEX 2014.

In legacy facilities and as buildings age, ensuring cGMP compliance can become complex. A review of a facility's gowning operations can bring insight into the current state of cGMP compliance. The author presents characteristics to look for and questions to ask.

Experimental work on wet granulation demonstrates the use of dynamic powder characterization to support continuous processing for tablet manufacturing.

Bioreactor for Mammalian Cell Culture

Gowning practices embody cGMPs and can be a tool to evaluate cGMP compliance.

Progress in equipment availability, process analytical technology, and advanced process control aids ongoing development of continuous solid-dosage manufacturing processes.

Printing, labeling, aggregation, and inspection equipment meets serialization requirements.

Players across the biologics value chain are attracted by the advantages of continuous biopharmaceutical manufacturing.

What challenges do biopharmaceutical manufacturers face when deciding to move from a fed batch process to a continuous process?

Challenges encountered when implementing a continuous monitoring system are reviewed.

sterilization approach-cycles controlled by differential pressure-is described.