
Optel Vision, a Canadian company specializing in track-and-trace and vision inspection solutions for manufacturing industries, announced that its facility in Limerick, Ireland is now opened to serve customers across Europe.

Optel Vision, a Canadian company specializing in track-and-trace and vision inspection solutions for manufacturing industries, announced that its facility in Limerick, Ireland is now opened to serve customers across Europe.

CDMO Vetter announced the addition of a new flexible serialization service, introduced in response to stricter packaging regulations calling for drugs to be serialized as a means to fight counterfeits.

Sartorius Stedim Biotech’s BIOSTAT A is a compact bioreactor and fermenter with accessible peristaltic pumps, probe ports, and supply connections.

Indian manufacturers are moving towards high-value, low-volume work, with complex chemistry and intellectual property challenges.

Dual sourcing is one of many possible solutions to securing the supply chain.

Solving the problem of tablet spots or specks involves prevention and thorough investigation.
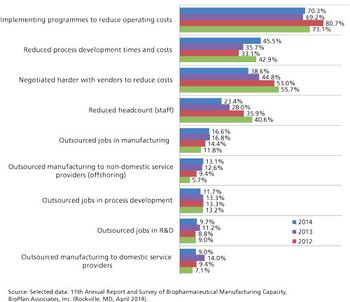
Outsourcing is taking on a greater role in the biopharmaceutical manufacturing industry.

Dalton Pharma Services announced it was awarded funding from ISTPCanada for its project to develop vaccines for respiratory syncytial virus and parainfluenza type 3.

The Baxter recall in the US of one lot of highly concentrated potassium chloride is due to a mislabeled overpouch.

The Singapore API facility will be operational by 2016.

The more than $200 million project will increase production capacity at the facility to support AstraZeneca':s maturing pipeline.

GSK Australia's Boronia site will install next-generation blow-fill-seal machinery for aseptic filling.

Over the last three weeks, we have been looking at each step and the benefit of adopting the process. This week, we look at Step 4, Measure.

New child-resistant packaging designs meet regulatory requirements, and consumer research into child-resistant closures continues.

Although the source of spots and specks on tablets is sometimes difficult to identify, following good maintenance and manufacturing practices can help solve or even prevent the problem.

A robotic dispensing system has an optional vision guided system.

The automated punch polisher for tablet tooling has double the capacity of the previous version for consistent, even polishing.

Robots are proving advantageous in filling, inspection, packaging, laboratories, and the manufacture of personalized medicine.

Grand River Aseptic Manufacturing has announced that the company executed two commercial production contracts in one day.

Advanced analytical methods are speeding up the targeted evaluation of potential viral contaminants.

Recent technology introductions demonstrate that accuracy, efficiency, and usability are top of mind for pharmaceutical industry professionals for analytical instruments.

Cryopreserved shipments are monitored with real-time tracking and intervention services.

As the second part of this series, we look at the importance of ?assessing? the punches and dies to check their condition and how this step may help avoid any problems that may occur during tablet manufacture.

Capsugel gives lead users access to intrinsically enteric capsule technology and support services.

Roche announced it will spend 450 million Swiss Francs on a new manufacturing facility in Suzhou, China.