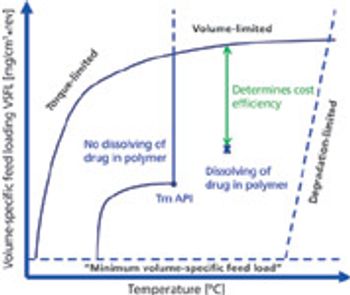
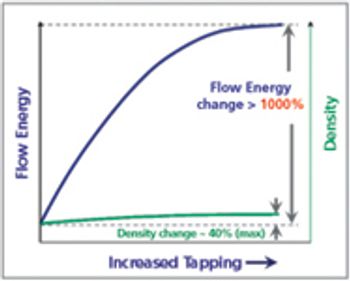
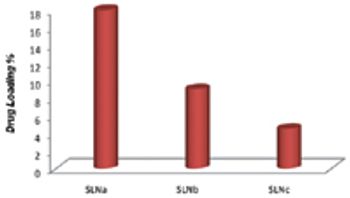
The physical form of an API is an important consideration in formulation development. Particle-engineering technologies, such as crystal design for controlling crystallisation and producing cocrystals, particle-size reduction and amorphous solid dispersions, help to optimise delivery of a drug.