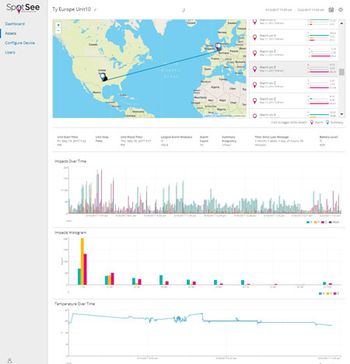
Considerations for monitoring impact, vibration, and temperature during package transportation and storage.

Considerations for monitoring impact, vibration, and temperature during package transportation and storage.

GE Healthcare launches Chronicle GMP-compliant automation software for cell therapy manufacturing.

The FactoryTalk Analytics LogixAI module from Rockwell Automation detects production anomalies.

Managing and controlling e-records is vital for maintaining CGMP data integrity.

Moving from paper-based to digitalized processes is the first step to enabling quality management and manufacturing to work in sync.

The GE Healthcare and Rockwell Automation collaboration will help meet the needs of the biopharma 4.0 era.

Katalyst D2D from ACD/Labs enables the design, planning, execution, and analysis of high throughput (HT) experiments. The web-based application was introduced at Pittcon 2019 in Philadelphia, PA.

The companies will join forces to improve gene- and cell-therapy manufacturing using the cloud and machine learning.

New technologies in the digital factory enhance quality, efficiency, and flexibility for bio/pharmaceutical manufacturing.

Eli Lilly and Company experts share the vision and the value of the digital plant for pharmaceutical manufacturing.

System connectivity and data analysis drive increased productivity in pharma manufacturing.

GS1, a global supply-chain standards organization, launched a new messaging standard in collaboration with GS1 US to help meet the requirements of the US Drug Supply Chain Security Act (DSCSA) for salable returns of serialized prescription drugs.
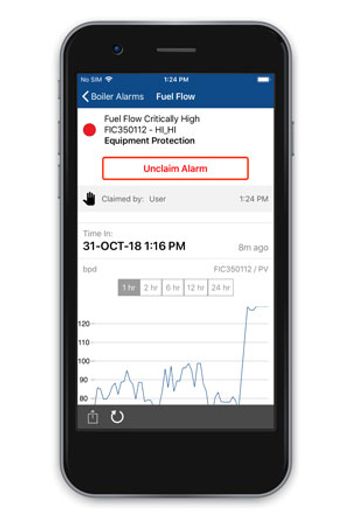
New alert customization and expanded Open Platform Communication support in Emerson’s DeltaV Mobile app improve notification and third-party system monitoring.

The Centre for Process Innovation (CPI) has led an Innovate UK project focused on the establishment of a generic framework and core capability to improve industrial productivity using computer models.

The Qualit-e Cloud web portal allows for secure uploading and sharing of quality documents for raw materials.

To improve production in pharmaceutical manufacturing, the IT, OT, and Quality functional groups must work together to get the most value from existing plant data.

Collaborative robots work beside laboratory employees to improve efficiency in pharmaceutical research and quality control labs.

Collaborative robots work beside laboratory employees to improve efficiency in pharmaceutical research and quality control labs.

Preventing problems in implementing a manufacturing execution system requires upfront analysis and detailed planning.

New software tools by Tecan, a provider of automated laboratory instruments and solutions, complement each other to enhance process monitoring of its liquid-handling platforms.

FDA grants support US research in continuous manufacturing monitoring and control techniques for bio/pharmaceutical manufacturing at Rutgers, MIT, and Georgia Tech.

Modern technologies, including Industry 4.0 and the Industrial Internet of Things, offer opportunities to increase biopharmaceutical manufacturing efficiency.

Siemens will become a preferred supplier for Sartorius Stedim Biotech’s automation solutions, and SSB will create a globally standardized automation platform for its biopharmaceutical manufacturing systems.

ABB’s modeling software, Shop Floor Integration V2.0, allows engineers to validate the connection of existing pharmaceutical manufacturing equipment with Werum’s PAS-X manufacturing execution system in a simulation prior to running it live.

Aerobic bioprocesses are highly dependent upon the oxygen transfer rate (OTR) from sparged to dissolved gas. The relatively low solubility of oxygen, however, makes the choice of mixer impeller configuration a critical design factor for the bioreactor vessel. This article describes a series of experiments and computational fluid dynamics (CFD) simulations to detail the effect of mixer configuration on the efficiency and effectiveness of a bioreactor vessel with respect to blend time and mass transfer.