
Data integrity in the analytical laboratory is an area of increasing focus for regulators such as FDA.

Data integrity in the analytical laboratory is an area of increasing focus for regulators such as FDA.

Pharmaceutical Technology spoke with Gloria Gadea-Lopez, associate director of Shire, and Martin Dittmer, PharmaSuite product manager at Rockwell Automation, about how manufacturing execution systems (MES) can improve productivity and the current challenges faced by companies implementing MES systems.

Presenters at the 8th Annual Forum on Manufacturing Execution Systems (MES) discuss how a vision and common terminology help companies implement MES globally.

Model-predictive design is applied to solid-dosage processes.
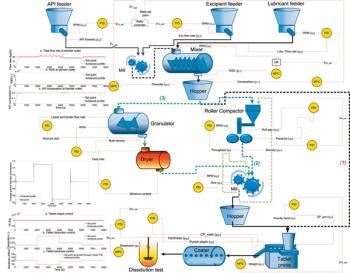
Applying model-predictive methods and a continuous process-control framework to a continuous tablet-manufacturing process.

Industry is moving toward closed-loop control of continuous processing.

Process analytical technology (PAT) is being successfully used to improve understanding and optimize pharmaceutical unit operations, but greater value can be obtained by integrating PAT with overall process control of a continuous manufacturing system. Pharmaceutical Technology spoke with Ivo Backx, manager of business and project development for the pharmaceutical industry at Siemens Industry Automation Division, to gain insight on the issues involved.

PharmTech Europe speaks to Bhaskar Sambasivan from Cognizant to find out more about social media, mobility and cloud computing.

Strategic management of intelligent devices is important in any processing field, and a new standards committee at the International Society of Automation aims to provide direction so that manufacturers can better utilize the devices' capabilities.
New product reviews for July 2012.

With social media receiving increased focus as the pharma industry strives to make the most of new communication technologies, we speak with Cognizant's Bhaskar Sambasivan to find out where pharma is when it comes to social media implementation and what more should be being done.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the May 2012 edition from Koslow Scientific Company and Vaisala.

Manufacturers seek clear path to develop safe-use approaches for more risky OTC therapies.

My network failed and I had to scrap my batch because my historian did not collect the required data. How do I upgrade the reliability of my network to maintain data continuity if my network fails again?

New product reviews for March 2012.

Guidance offered on how to deal with off-label information requests.

We interview Subhro Mallik from IT firm Infosys about how pharma is responding to the cloud computing phenomenon and what more can be done to realise business benefits.

Implementing a zero-waste strategy is not merely an option, it can be seen as a crucial business imperative.
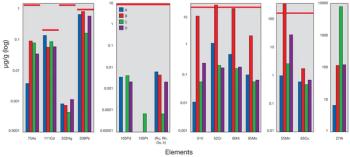
The US Pharmacopeia (USP) proposes to lower the maximum permissible limits of trace elements in pharmaceuticals and recommends that impurities be measured through automated instrumentation-based methods. The proposed regulations specify inductively coupled plasma–mass spectrometry (ICP–MS) and inductively coupled plasma–optical emission spectrometry (ICP–OES) as the techniques of choice. This article discusses the benefits of ICP–MS and ICP–OES for the accurate detection of trace elements in pharmaceutical products, in compliance with the proposed USP chapters.

The authors discuss the analytical methods and related testing for bioequivalence studies of ADCs.

A Technical Forum Moderated by Patricia Van Arnum, featuring contributions from PerkinElmer, BioTools, Chiral Technologies, Shimadzu Scientific Instruments, GE Analytical Instruments, and Waters.
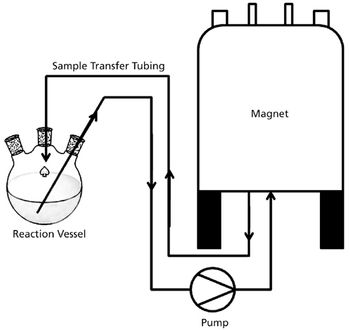
The authors describe the benefits of nuclear magnetic resonance (NMR) compared with traditional monitoring techniques. They also discuss how NMR reaction monitoring provides a new process analytical technology tool for industry.

Dr. Fred Jordan, CEO at AlpVision SA, explains how web-based server solutions can aid anticounterfeiting strategies and why such systems are seeing increased uptake.

GE Healthcare, the health business of General Electric, will dedicate $1 billion of its total R&D budget during the next five years to its technologies for manufacturing biopharmaceuticals and for cancer research. Part of the money will go toward expanding the company's cancer-diagnostic and molecular-imaging capabilities, as well.
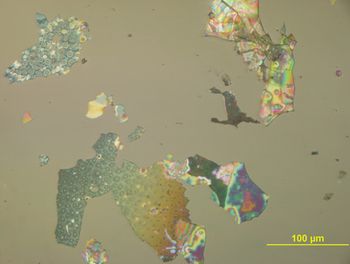
Glass flaking or delamination can result in a failed quality-assurance test, thus bringing production to a halt and causing substantial revenue loss. If glass delamination remains undiscovered, it can pose a serious contamination risk to the drug product and a potential health risk to the public.