
FDA wants industry to talk to them about the science underlying process innovations-really.

FDA wants industry to talk to them about the science underlying process innovations-really.

Chemocatalysis and biocatalysis are important elements of an effective strategy for improving yield and stereoselectivty.

The growth of Brazil's generic-drug market is on a fast track, but what are the projections for the sector's future?

A Pharma Business Imperative.

When vessels, seals, and cooling units go haywire, operators must get in the mix.

A Technical Forum featuring representatives from Dow Chemical, ISP, and DMV-Fonterra Excipients. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The author describes the new IPEA excipient good-manufacturing-practice certification program that is now ANSI accredited. This article is part of a special issue on excipients and solid dosage.

The authors review new regulatory expectations and describe potential approaches to accommodate excipient variability. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The authors formulated bupropion hydrochloride tablets with various grades of methacrylic copolymers and analyzed the properties of the resulting dosage forms. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

Why spray drying processes offer a range of particle engineering possibilities.

The pharmaceutical industry must understand its responsibilities to improve the safety of chemicals as defined by the REACH initiative.

Charles River Acquires WuXi AppTec; Amgen Appoints President and COO; and More.

The Society of Chemical Manufacturers and Affiliates is raising concerns over a recently introduced bill in the US Senate, which seeks to reform the Toxic Substances Control Act.

FDA Issues Warning Letters to Astellas, GSK, And Novartis; Sandoz Acquires Oriel Therapeutics; And More.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the April 2010 edition from Camfil Farr and K-Tron.

Cephalon Completes Acquisition of Mepha; Xcellerex Appoints President and CEO; and More.

Eli Lily Wins Court Battle; Genzyme Appoints COO; And More.

As the industry continues to evolve, do we know where we're headed?

Laboratory personnel share interesting tales as well as stories of unexpected tails.
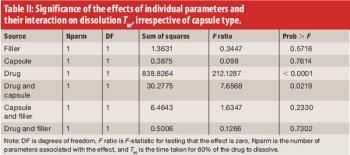
The authors examined the disintegration and dissolution profiles of propranolol and rofecoxib tablets overencapsulated with standard hard-gelatin capsules and with capsules specifically designed for double-blind clinical trials.

As demand for global vaccine development and production grows, all eyes are turning to Asia.

Interphex Showcase 2010.

The BIO convention, and healthcare reform, could re-energize biotech.

Pharmaceutical Technology's annual survey on equipment and machinery reveals the spending levels and types of spending made in 2009 and planned for 2010.

Growth in the market for monoclonal antibodies, recombinant proteins, and vaccines creates new opportunities for drug companies and suppliers.