
Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

The authors developed a metronidazole-based floating drug-delivery system to investigate the effect of rate-controlling polymers on release pattern and duration of buoyancy in matrix tablets.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.

The last in a series of eight case studies from the Product Quality Research Institute focuses on internal GMP audits.
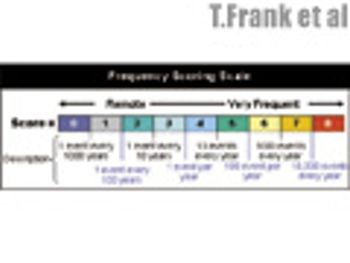
The sixth in a series of eight case studies from the Product Quality Research Institute focuses on packaging line GMP optimization.

Researchers develop various catalytic approaches for improving yield, purity, stereoselectivity, and process conditions.
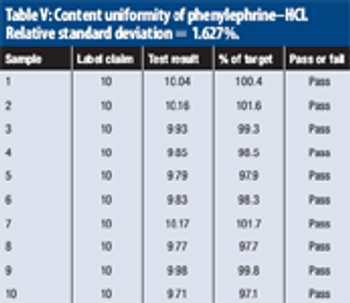
The study evaluates near-infrared analysis of tablets nominally containing 4 mg of chlorpheniramine maleate and 10 mg of phenylephrine hydrochloride per dose.
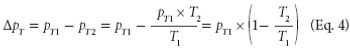
The authors describe the operational qualification of test accuracy with regard to temperature drift using a thermal-compensation algorithm on several freeze dryers.
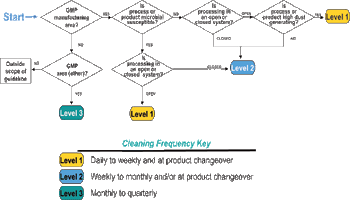
The fifth in a series of eight case studies from the Product Quality Research Institute focuses on nonsterile facility cleaning requirements.

John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.

The authors describe the concept of the limiting discriminatory the limiting discriminatory threshold (LDT) as an objective means of evaluating the inherent quality requirement of a large-sample content-uniformity test.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

This risk-management case study focuses on assessing empty capsules.

Where are the new excipients, the new solubilisers and sustained release excipients?
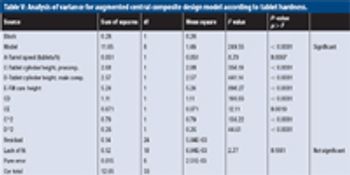
The author prepared and analyzed a detailed design of experiments for the manufacture of a simple tablet formulation. The aim was to test whether tablet hardness and weight could be controlled during the compression process by adjusting certain machine parameters.

Internal and external Web-based communities are changing how pharma companies can innovate.

The author offers perspectives on ways in which pharmaceutical companies and other stakeholders in the supply chain can confront the threat of counterfeit products, cargo theft, illegal diversion, and economically motivated adulteration.

Might European officials reverse their position on acceptable production methods?
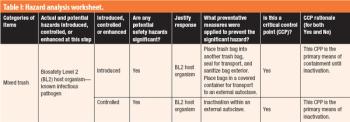
The third in a series of eight case studies from the Product Quality Research Institute focuses on facility biocontainment and inactivation.

Biocatalysis, chemocatalysis, and other chiral technologies continue to attract the investment dollars of CMOs and fine-chemical companies.

Direct dosing APIs during R&D studies can reduce the overall testing time of a drug candidate by allowing for a greater throughput of compounds through the R&D department.

The increasing cost of crucial manufacturing input factors, such as energy and raw materials, has been a severe threat to several companies.

Elham Blouet from Roquette explains the importance of carbohydrates for injection and the challenges in this niche market.
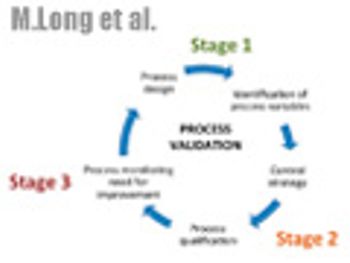
The authors desribe the three-stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation.