
Listen to roundtables from the 2011 ExcipientFest/IPEC conference, addressing developing issues in excipient functionality and continuous manufacturing.

Listen to roundtables from the 2011 ExcipientFest/IPEC conference, addressing developing issues in excipient functionality and continuous manufacturing.
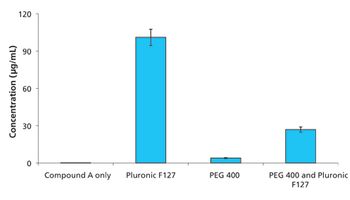
The study results suggested that Pluronic F127 might be a potent inhibitor of drug precipitation for Labrasol formulations.

India's drug pricing authority increased the retail costs of certain domestically manufactured drugs, but not those of imported drug products. Pharma is asking why.

Effective containment in API and drug-product manufacturing encompasses a variety of process, equipment, and operational issues.

Takeda Pharmaceutical has agreed to acquire Nycomed, a Swiss pharmaceutical company for $13.5 billion, excluding Nycomed's US dermatology business. The deal is intended to increase Takeda's presence in European and emerging markets.

The Office of the United States Trade Representative issued a report as part of its annual review of the global state of intellectual-property rights protection and enforcement.

The IPEC is soliciting public comment about a draft plan for the independent certification of manufacturers and suppliers of pharmaceutical excipients.

Eastern Europe's pharmaceutical leader, Hungary, is working to maintain its number-one status while also pursuing new avenues, especially in biopharmaceuticals.

Approaches in using small-molecule and peptide synthesis offer promise in widening the scope of drug candidates.

GlaxoSmithKline (GSK) identified certain over-the-counter (OTC) brands in its consumer healthcare business that the company plans to divest.

Axcan acquires Mpex Pharmaceuticals; Bend Research receives patent for improving bioavailability of low-solubility drugs; and More.

FDA recently published guidance for preventing the cross-contamination of finished pharmaceuticals and active pharmaceutical ingredients with nonpenicillin beta-lactam antibiotics.

Merck & Co. has formed a joint venture with the Mumbai-based specialty pharmaceutical company Sun Pharmaceutical Industries to develop, manufacture, and commercialize new combinations and formulations of branded generic drugs in emerging markets.

The contract-research industry in China is growing in leaps and bounds, and Big Pharma is leading the way.
Chemocatalytic and biocatalytic routes play an important role in improving the manufacture of intermediates and active pharmaceutical ingredients.

The Bulk Pharmaceutical Task Force (BPTF) of the Society for Chemical Manufacturers and Affiliates (SOCMA) and the European Fine Chemicals Group (EFCG) of the European Chemical Industry Council (CEFIC) are calling on FDA to mandate inspections of foreign active pharmaceutical ingredient (API) manufacturing sites with the cost borne by those sites being inspected. Both organizations have indicated a willingness to pay fees for these inspections when performed on their member-owned facilities that are located outside the United States.

Fujifilm and Merck & Co. have formed a definitive agreement by which Fujifilm will acquire the Merck BioManufacturing Network, a contract biopharmaceutical manufacturing and development business of Merck.

Pfizer Completes King Acquisition; ViiV Healthcare Names Chairman; and More.

Brazil develops its first national plasma fractionation plant.

Q&A with Robert Hardy, chief executive of Aesica

The power of emerging markets is reflected in the pharma's sales and production positions.

INTERPHEX 2011 aims to address the industry's unique characteristics.

The authors outline the key decision points FDA must consider in putting forth a US regulatory pathway for biosimilars.

This article is Part I of a three-part series on biopharmaceutical issues in public health, government, and developing-world markets. Part 1 focuses on drug-development. Part II, which examines manufacturing, appeared in the April 2011 of Sourcing and Management. Part III, which analyzes distribution and administration,appeared in the May 2011 issue.

Contract manufacturers and fine-chemical suppliers announce capacity expansions and service enhancements of Informex.