
Policymakers weigh new rules to ensure the safety and quality of drugs made with tiny particles.

Policymakers weigh new rules to ensure the safety and quality of drugs made with tiny particles.

Optimizing the solid form of a drug reaps scientific and technical awards.

A well-balanced guide to industrial bioseparations provides valuable information.
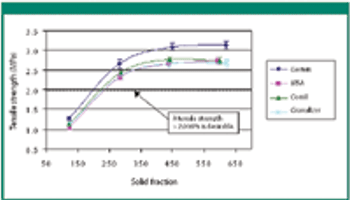
The authors evaluate the effect of various mill types on particle-size distribution, flowability, tabletability, and compactibility.
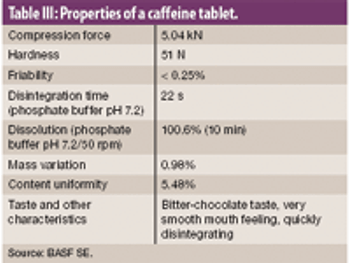
The authors examine the effectiveness of an excipient comprised of mannitol, polyvinyl acetate, and crospovidone using model actives loperamide hydrogen chloride and caffeine.
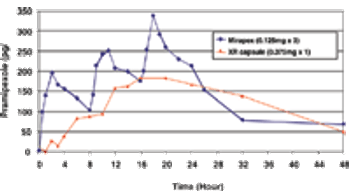
Novel hydrophobic bioadhesive polymers and dosage designs are now available to effectively achieve tailored release kinetics of a broad range of drugs to meet the clinical needs.

Pharma companies could benefit from the lessons learned in this fall's financial crisis.
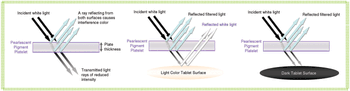
Sophisticated excipient development, especially for coatings, is staying on top of new challenges and meeting expanding industry needs.

IPEC Chairman Dave Schoneker discusses current efforts toward facilitating regulatory reviews of new excipients.

Pharmaceutical Technology will feature video coverage of AAPS this month.

Molecules called "chaperones" facilitate correct protein folding.
Rapid advances in drug discovery have led to the identification of a number of compounds with good therapeutic potential.

During the past two decades, regulations have evolved in both Europe and the US to accommodate the technological developments in the pharmaceutical, biotechnology and medical device industries.

The US Food and Drug Administration has awarded the National Institute for Pharmaceutical Technology and Education (NIPTE), a not-for-profit organization comprising 11 universities, a $1.19 million contract to develop quality by design (QbD) guidance elements for design space and scale-up of unit operations.

Also, Maxygen looks to costs, jobs; Receptor BioLogix appoints Dale R. Pfost CEO; more...

Pfizer and UCB formed a technology company named Cyclofluidic with the aim of accelerating the drug-discovery process.

Also, MedImmune opens Cambridge, UK, facility and makes reverse engineering pact with Omninvest; BD Medicine appoints Carol Adiletto VP of clinical and regulatory affairs; more...

Also, Millipore opens new membrane-casting manufacturing facility in Ireland; Surface Logix appoints Keith Dionne president, CEO, and a member of the board; more...

Also, Merck & Co. discontinues development of its obesity drug taranabant; Synthetech names Frederic Farkas director of manufacturing; more...

The European Fine Chemicals Group (EFCG) and the International Pharmaceutical Excipents Council of Europe (IPEC Europe) announced the formation of a European Pharmaceutical Excipients Certification Project to develop advocacy and stakeholder management in Europe and to give advice to two European working teams as part of an effort to develop a certification program for manufacturers and distributors of pharmaceutical excipients.
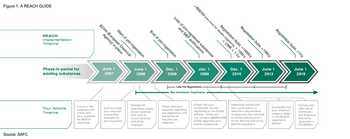
The pharmaceutical industry faces compliance with the REACH regulation, the European Union's regulation on chemicals and their safe use. Fully understanding the requirements, achieving compliance, and developing strategies for working with suppliers are key for avoiding interruption of the supply chain.
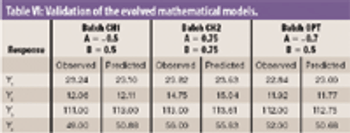
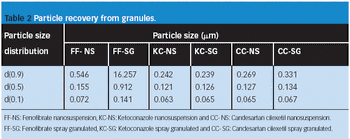
The authors studied the effect of the combination of binders on the flow and compressibility characteristics of the agglomerates of binary combination of lactose and dibasic calcium phosphate dihydrate.
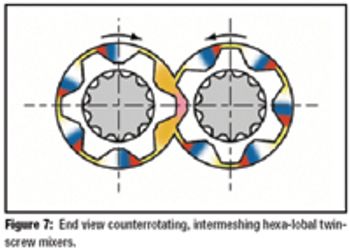
The author describes the benefits, processes, and practicality of using hot-melt extrusion to mix active pharmaceutical ingredients with pharmaceutical-grade polymers.

Advancements add yet another challenge for industry's already overextended regulatory body.

This article summarizes the classification systems for topical liquid and semisolid dosage forms used for dermatological application and notes some differences between FDA and USP classification.