
At first glance, the title of this article may bring a wry smile to the face of many an astute practitioner, but I can provide 'documentary evidence' that free validation is not a just a play on words, but a financial reality.

At first glance, the title of this article may bring a wry smile to the face of many an astute practitioner, but I can provide 'documentary evidence' that free validation is not a just a play on words, but a financial reality.
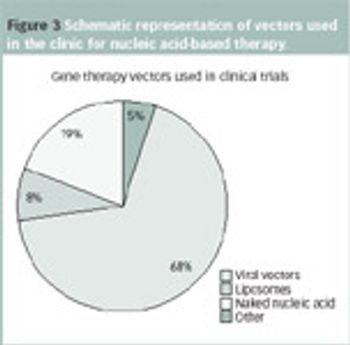
The introduction of biomolecules into cells is a key technology for research in biological sciences.

How can any company be sure that the standards that suppliers might claim to operate, and might be able to demonstrate from time to time, are actually being practised all the time?

The US Pharmacopeia (USP) posted revised monographs for heparin sodium and heparin calcium on its website.

Also, Stiefel Laboratories will acquire Barrier Therapeutics, Danube Pharmaceuticals appoints Brian Levy COO, more...

Also, Patheon to expand Puerto Rico facility, Creabilis Therapeutics appoints Tony Wilson CEO, more...

Effective July 1, 2008, the Office of Generic Drugs (OGD) will require abbreviated new drug applications (ANDAs) to include data that demonstrate the manufacturer's control of residual solvents.

Also, Shire voluntarily recalls ADHD patch Daytrana

Also, Pall plans expansion in South America, Anthony Clarke joins Alexza Pharmaceuticals, more...

The US Food and Drug Administration advised patients, caregivers, and healthcare professionals to switch to hydrofluoroalkane-propelled albuterol inhalers now because chlorofluorocarbon-propelled inhalers will not be available in the US after Dec. 31, 2008.

Antibody drug conjugates offer a niche opportunity in drug development and contract manufacturing.
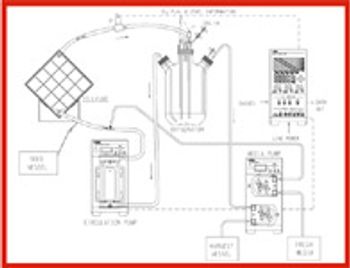
Creating a kinder, gentler manufacturing process that doesn't kill the product is the goal of process developers doing large-scale cell culture for cell therapy.

Nanoparticle-based systems present many advantages for the delivery of current and emerging biological drugs.

Letting the public inside the drug development process may increase their faith in what we do.

The good, the bad, and the ugly about direct-to-consumer advertising.

Scientists are uncovering signaling systems that operate via cannabinoid messenger molecules.

An ambitious survey of characterization techniques presents current information.
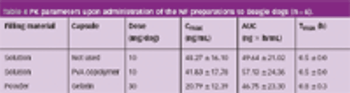
When drugs are encapsulated, electrification (the electrostatic charge of the capsule) may sometimes cause problems, such as capsule adhesion during transportation or dispersion of the capsule content in the filling process.

Also, GVK BIO and Wyeth Pharmaceuticals form research agreement, Eli Lilly announces changes to management, more...

The European Federation of Pharmaceutical Industries and Associations (EFPIA), the trade association representing European pharmaceutical manufacturers, issued recommendations to the public consultation launched in March by the European Commission's proposed drug anticounterfeiting measures. EFPIA's proposal includes a ban on drug repackaging.

Also, Pfizer to close Indiana "Exubera" facility, executive appointments at Patheon, more...

Also, Quintiles Transnational to acquire Eidetics, ChemAxon appoints Alex Drijver CEO, more...

Also, FDA removes OAI status for Watson's Florida facility, executive management changes as GSK, more...

Polyplus-transfection, a company that researches, develops, and commercializes drug-delivery solutions for biomolecules, created a new technology designed to enhance in vivo delivery of small interfering RNAs (siRNAs) when they are associated with a cationic polymer.
Scientists are giving up on a preventive vaccine for AIDS, but there are lessons to be learned.