
The authors demonstrate that sustained-release delivery can help avoid the risk of sudden higher-blood concentration of a drug to avoid toxicity.

The authors demonstrate that sustained-release delivery can help avoid the risk of sudden higher-blood concentration of a drug to avoid toxicity.

The growth of Brazil's generic-drug market is on a fast track, but what are the projections for the sector's future?

A Pharma Business Imperative.

Agreement on standards for excipient qualification, development, and fair pricing is underway.

When vessels, seals, and cooling units go haywire, operators must get in the mix.

A Technical Forum featuring representatives from Dow Chemical, ISP, and DMV-Fonterra Excipients. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The author describes the new IPEA excipient good-manufacturing-practice certification program that is now ANSI accredited. This article is part of a special issue on excipients and solid dosage.

The authors review new regulatory expectations and describe potential approaches to accommodate excipient variability. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

The author proposes techniques, based on Six Sigma methods, for monitoring such processes to discover their airflow patterns and reduce opportunities for spillage.This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

A Q&A with the First Federation Chair, Patricia Rafidison.

The authors formulated bupropion hydrochloride tablets with various grades of methacrylic copolymers and analyzed the properties of the resulting dosage forms. This article is part of PharmTech's supplement "Solid Dosage and Excipients 2010."

Testing the dissolution rates of a pharmaceutical formulation (also known as in vitro availability) aids drug quality control and is a compulsory requirement of the British, European and US pharmacopoeias.

Charles River Acquires WuXi AppTec; Amgen Appoints President and COO; and More.

FDA Issues Warning Letters to Astellas, GSK, And Novartis; Sandoz Acquires Oriel Therapeutics; And More.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the April 2010 edition from Camfil Farr and K-Tron.

If you’ve worked in the drug industry for a while, chances are good that you remember Pfizer’s Rezulin, which was produced through hot melt extrusion (HME). If you work for a major pharmaceutical manufacturer, it’s likely that your company owns a twin-screw extruder. Yet HME has not been a common way of manufacturing drugs, and many industry employees don’t understand the principles of HME or the advantages that the technique offers.

Cephalon Completes Acquisition of Mepha; Xcellerex Appoints President and CEO; and More.

Eli Lily Wins Court Battle; Genzyme Appoints COO; And More.

As the industry continues to evolve, do we know where we're headed?

Laboratory personnel share interesting tales as well as stories of unexpected tails.
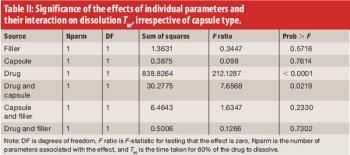
The authors examined the disintegration and dissolution profiles of propranolol and rofecoxib tablets overencapsulated with standard hard-gelatin capsules and with capsules specifically designed for double-blind clinical trials.

As demand for global vaccine development and production grows, all eyes are turning to Asia.

Interphex Showcase 2010.

The BIO convention, and healthcare reform, could re-energize biotech.

The authors describe the origins of single-use components and explain their application to aseptic processes. They also show how disposable devices have changed over time and offer a glimpse of the future.