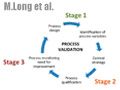
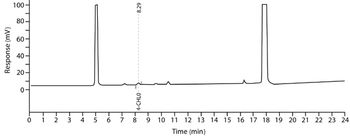
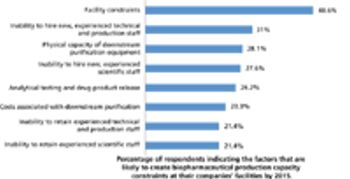
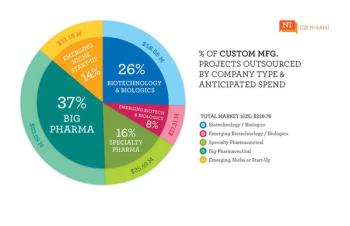
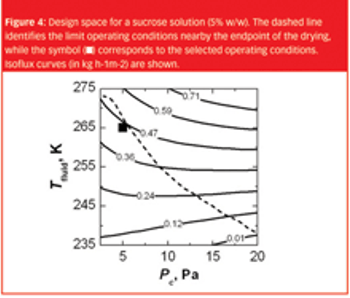
A Q&A with Joe Cascone, director of potent pharmaceutical development at Metrics, moderated by Patricia Van Arnum. Discussion of the key considerations made in facility design, equipment selection, and operations to achieve desired levels of containment.