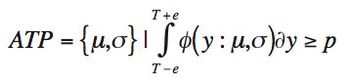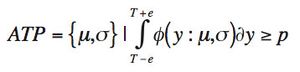Articles by Melissa Hanna-Brown
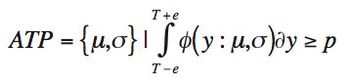
Using Quality by Design to Develop Robust Chromatographic Methods
ByStephen Colgan,Jackson Pellett,Melissa Hanna-Brown,Gregory Sluggett,Tim Graul,Loren Wrisley,Gregory Steeno,Jim Morgado,Roman Szucs,Brent Harrington,Kimber Barnett The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods.