
The authors investigate the influence of hydro-alcoholic media on hydration and drug release from polyethylene oxide extended-release matrices.

The authors investigate the influence of hydro-alcoholic media on hydration and drug release from polyethylene oxide extended-release matrices.
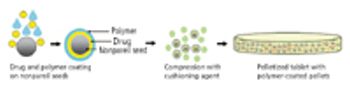
The current review describes the role and selection of excipients, pellet core, coating materials, and compression with various cushioning agents.

Two popular methods for detecting protein aggregates are analytical ultracentrifugation (AUC) and size-exclusion chromatography?multiangle light scattering (SEC?MALS). These techniques? results correlate relatively well, but each one has its own strengths.

Listen to roundtables from the 2011 ExcipientFest/IPEC conference, addressing developing issues in excipient functionality and continuous manufacturing.

Can microdosing make medicines safer and more effective for children?
More sophisticated biological expression systems expand the functionality of the traditional systems for protein synthesis.

A recent industry survey shows keen interest in improving bioreactors and cell-culture media.
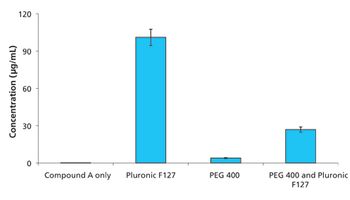
The study results suggested that Pluronic F127 might be a potent inhibitor of drug precipitation for Labrasol formulations.

Sometimes, there are just too many cooks in the kitchen.

Effective containment in API and drug-product manufacturing encompasses a variety of process, equipment, and operational issues.
BASF's Jan Bebber explains why immediate-release formulations boost drug efficacy and aid patient compliance.

The rising popularity of fast dissolving, immediate-release dosage forms can be attributed to their convenience and ease of administration.

Teva Pharmaceutical Industries agreed to pay shareholders $460 million in cash to acquire a 57% stake in Taiyo Pharmaceutical Industry. Teva also will offer to buy all outstanding shares of Taiyo.

The Office of the United States Trade Representative issued a report as part of its annual review of the global state of intellectual-property rights protection and enforcement.

PhRMA Urges Congress to Reauthorize Legislation for Pediatric Drugs.

The IPEC is soliciting public comment about a draft plan for the independent certification of manufacturers and suppliers of pharmaceutical excipients.

Eastern Europe's pharmaceutical leader, Hungary, is working to maintain its number-one status while also pursuing new avenues, especially in biopharmaceuticals.
The authors examine the influence of glass-transition temperature, melt viscosity, degradation temperature, and process settings.

Emerging methods could provide alternative ways of producing inhalable drug particles.

Approaches in using small-molecule and peptide synthesis offer promise in widening the scope of drug candidates.

Nanosponges, a controlled-release nanoparticle system, shows promise in targeted drug delivery

Can the semiconductor industry help Big Pharma develop therapies?

Many factors affect research results.
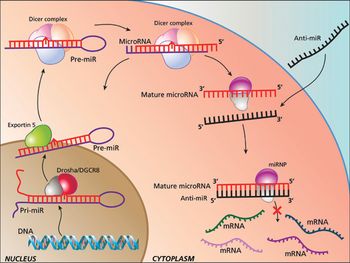
The authors provide further insight into microRNA biology, and the simplicity of anti-miR oligonucleotide drug delivery.

The author describes recent developments to help overcome the downstream processing bottleneck. This article is part of a special issue on Sterile Manufacturing and Bioprocessing.