
It's time to maintain a thorough traceable excipient trail.
Arthur J. Falk, PhD, is president and CDO of International Pharmaceutical Excipient Auditing (IPEA).

It's time to maintain a thorough traceable excipient trail.
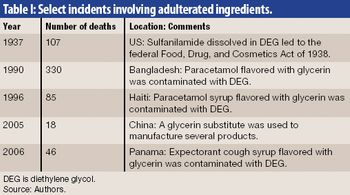
Securing the integrity of the excipient supply chain is a crucial task in ensuring the overall pharmaceutical supply chain. The authors outline excipient-control strategies and practices for the manufacture, distribution, and receipt of excipients.

Two FDA final rules issued in response to the Bioterrorism Response Act of 2002 may significantly affect the supply and importation of pharmaceutical excipients.

Published: March 2nd 2010 | Updated:
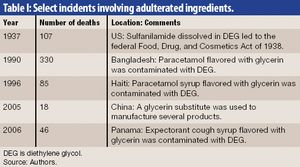
Published: October 1st 2008 | Updated:

Published: January 2nd 2004 | Updated: