
Darmstadt, Germany (May 13)-Mylan Laboratories Inc. and Merck KGaA have signed a share purchase agreement under which Mylan will acquire all of Merck's worldwide operations within Merck Generics, the company?s generics business.

Darmstadt, Germany (May 13)-Mylan Laboratories Inc. and Merck KGaA have signed a share purchase agreement under which Mylan will acquire all of Merck's worldwide operations within Merck Generics, the company?s generics business.

Rockville, MD (May 1)-The US Food and Drug Administration issued a report, "Critical Path Opportunities for Generic Drugs," to identify the scientific challenges, including those in manufacturing science, in developing generic drugs and the opportunities for collaborative solutions in resolving those challenges.

Washington, DC (April 30)-Less than two weeks after the Senate Health, Education, Labor, and Pensions Committee voted to reauthorize the Prescription Drug User Fee Act (PDUFA), the bill this week moved onto the Senate floor.

Geneva, Switzerland (Apr. 27)-A meeting of the World Health Organization and the Committee for Medicinal Products for Human Use's approval of Novartis's new cell culture-derived influenza vaccine offered new hope that sufficient numbers of vaccines could be produced in case of a pandemic.
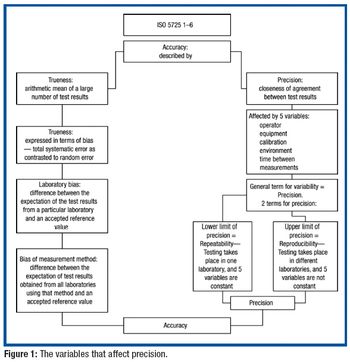
USP applies metrological principles to the dissolution procedure alone and in collaborative studies to understand and minimize potential sources of variability.

There is a tremendous need to enhance delivery of potential therapeutics to the brain for treatment of central nervous system (CNS) disorders. The blood brain barrier (BBB) restricts and controls the exchange of compounds between the CNS and the blood, which requires discovery of new modalities allowing for effective drug delivery to the CNS. Polymer nanotechnology has now become one of the most attractive areas of pharmaceutical research. This review focuses on the current progress in polymeric nanoparticles, where the specific arrangement of the polymeric matter at the nanoscale is utilized to design drug delivery systems that provide safe and efficient transport of CNS drugs across the BBB.

This article discusses a number of factors that may influence the behaviour of conjugated biopharmaceuticals. Optimizing bioconjugation processes may be critical to achieve the desired drug performance.

One of the major concerns with introducing PAT, however, is that the bias towards process engineering may not ultimately lead to complete control of product quality.

...Scotland has a favourable regulatory environment, funding support from its government and some of the most advanced research facilities in the world.

Irradiation is an established method of sterilization for pharmaceutical products. Radiation sterilization can be achieved with gamma rays, electron beams, and X-rays. Each of these techniques has its advantages and disadvantages. The author describes these methods, the ways to find the correct sterilization doses, and the regulatory and safety concerns about irradation sterilization.

Aseptic processing has advanced over the past several decades, yet the pharmaceutical industry is still accepting of its limitations, particularly as it relates to human intervention as a source of contamination. The authors explain the importance of further diminishing the role of operators in aseptic processing and the approaches and technologies needed to achieve that goal.

Interphex2007, New York, NY (Apr. 25)-As governments begin to contemplate the possibility of biological terrorism or a pandemic event, a new problem begins to emerge: in the case of a pandemic or an attack, even if a vaccine or treatment exists, how could it be produced in sufficient numbers to prevent the deaths of millions of people? That question was addressed in at the conference session, "Responding to Bioterrorism and Pandemic Events: A Case for Development of Flexible Manufacturing Space for Vaccine Production," at Interphex today.

Lyon, France (Apr. 17)-Sanofi Pasteur, the vaccine division of the Sanofi-Aventis Group, announced that the US Food and Drug Administration has licensed its H5N1 vaccine, making it the first avian-influenza vaccine for humans in the United States.
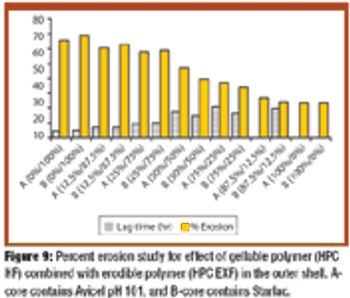
The authors prepared and tested press-coated tablets with various weight ratios of ethylcellulose to hydroxypropylcellulose (HPC) and various ratios of two different batches of HPC as an outer coating shell and fillers in core tablets. The tablets were examined for changes in time lag and release patterns of salbutamol sulfate.
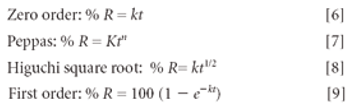
Hydrogels are biocompatible drug delivery systems by which the physical properties can be controlled by the cross-linking density. Hydrogels were prepared by copolymerization of acrylic acid monomers in the presence of poly(ethylene glycol)(PEG) to form polyethylene diacrylate (PEDGA). Various molecular weights of PEGs were used for the synthesis of PEGDA to study the effect of molecular weight of PEG on the properties of hydrogels. These hydrogels were further characterized for free water, swelling behavior, water diffusion, drug loading, and drug release profile. By analyzing the swelling behavior and release pattern of the hydrogels, the authors show that these systems can be suitably used for controlled delivery of drugs.
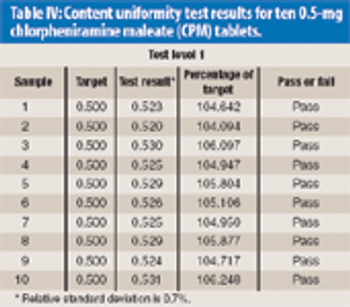
Near-infrared (NIR) assay and content uniformity of tablets provide fast, accurate means of monitoring tablet production that are in step with FDA's process analytical technology initiative.The authors discuss the process for testing a newly released NIR tablet analyzer to determine instrument precision and accuracy using chlorpheniramine maleate tablets.The data show promising results that could relieve laboratory workload of high-performance liquid chromatography analysis and bring analysis closer to real time for process monitoring.

A wealth of citations helps illustrate the applicability of the analytical methods and also highlights their pitfalls.

... there is a huge manufacturing challenge involved in bringing cell therapies (especially stem cell therapies) from the lab bench into the clinic.
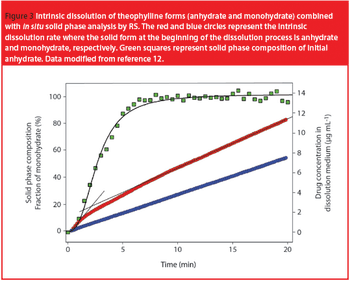
When a dosage form or an API is introduced to the gastrointestinal tract, or dissolution media mimicking it, a transformation from a metastable to a more stable form with lower solubility and bioavailability is possible.



Gurgaon, Haryana, India (Mar. 13)-Ranbaxy Laboratories Limited confirmed that it has made a nonbinding bid for the generic drug business of Merck KGaA.

Just because the wheels are turning doesn't mean they're going forward.

The book is written for drug-delivery scientists experienced in the dermatological or transdermal fields.

Washington, DC (Feb. 22)-The Biotechnology Industry Organization criticized two separate studies respectively released by the Pharmaceutical Care Management Association and Express Scripts, Inc. regarding the cost-savings, interchangeability, and market penetration of follow-on biologics.