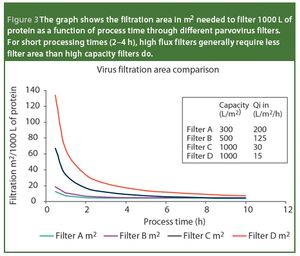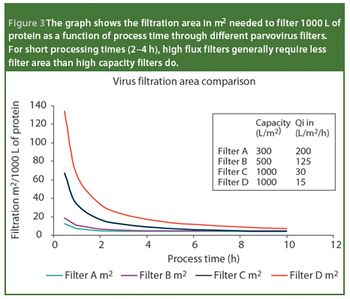
Virus safety of biotech- and plasma-derived therapeutics is ensured through complementary manufacturing and quality control measures that include the control and monitoring of raw materials, the validation and implementation of effective virus clearance technology and the monitoring of final filled product for the presence of virus. Virus filtration, which is considered a robust and effective virus clearance technology, is a common unit operation in the manufacture of biologicals. In this article, we review the points that must be considered when selecting a virus-retentive filter. The areas covered include regulatory considerations; selecting, optimizing and validating a virus filtration step; and process scale implementation - areas that are critical to users of virus filters.