
Basel, Switzerland (June 6)-Roche, in an agreement with the European Medicines Agency and the Swiss Agency for Therapeutic Products, recalled all batches of "Viracept" (nelfinavir) powder and tablets in Europe and some other regions of the world.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Basel, Switzerland (June 6)-Roche, in an agreement with the European Medicines Agency and the Swiss Agency for Therapeutic Products, recalled all batches of "Viracept" (nelfinavir) powder and tablets in Europe and some other regions of the world.

Rockville, MD (May 31)-The US Food and Drug Administration issued a draft guidance on how to design bioequivalence (BE) studies for specific drug products to support abbreviated new drug applications (ANDAs).

Rockville, MD (May 31)-The US Food and Drug Administration finalized guidances for seasonal and pandemic influenza vaccines, outlining the regulatory pathways for developing and approving these products.
Pharma companies and CMOs build positions in mAbs.

Nanotechnology is emerging as a tool for resolving challenges in delivering poorly water soluble and highly potent drugs.
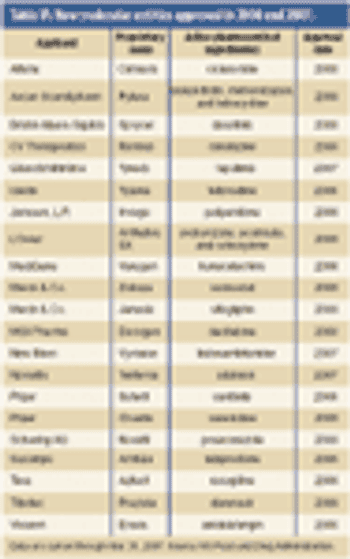
Pharmaceutical Technology's Annual Manufacturers' Rankings provides perspectives on revenues, product positioning, R&D spending, pharmaceutical manufacturing activity, and capital projects of the major drug companies.

Reflecting strong growth prospects for certain biologic-based drugs, the biotechnology and pharmaceutical majors are proceeding with a strategy of expanding their internal manufacturing networks and partnering with select contract manufacturing organizations (CMOs).

Boston, MA (May 8)-The global biotechnology industry showed several positive signs in 2006, including increases in overall revenues and financing, although the industry as a whole continues to operate at a loss, according to Ernst & Young's annual analysis of the biotechnology industry.

Rockville, MD (May 1)-The US Food and Drug Administration issued a report, "Critical Path Opportunities for Generic Drugs," to identify the scientific challenges, including those in manufacturing science, in developing generic drugs and the opportunities for collaborative solutions in resolving those challenges.

Devens, MA, Montes Claros, Brazil, and Florence, SC (May 2)-Bristol-Myers Squibb Company, Novo Nordisk A/S, and Roche provided updates to their companies? respective manufacturing expansions.

Rockville, MD (May 1)-The US Food and Drug Administration issued a guidance to alert pharmaceutical manufacturers, pharmacy compounders, repackers, and suppliers to the potential public health hazard of glycerin contaminated with diethylene glycol (DEG), a poison.

The pharmaceutical majors build their capabilities in peptide technology, and contract manufacturers expand to meet growing demand for bulk peptides.

Ricerca Biosciences LLC a contract drug-development company, is embarking on a growth strategy upon which it hopes to double its revenues by 2009. The company recently completed a financial restructuring that includes new equity and term facilities totaling nearly $50 million. That financing will enable Ricerca to implement a multiyear business plan, which includes a $15-million investment to add chemistry and biological laboratories to its site in Concord, Ohio.

Basel, Switzerland (Apr. 26)-Roche outlined advances it is production strategy for "Tamiflu" (oseltamivir) for treating the H5N1 avian influenza virus.

London (Apr. 23)-AstraZeneca PLC agreed to acquire MedImmune, Inc. for approximately $15.6 billion. The deal is expected to close in June 2007.

Interphex2007, New York, NY (Apr. 26)-Robustness studies for analytical methods are critical in being able to provide the assurance to the quality of an analytical method, a topic addressed in a conference session, "Performing Analytical Method Validation Robustness for Regulatory Compliance," at Interphex on Thursday.

Interphex2007, New York, NY (Apr. 26)-Maintaining reliable equipment in pharmaceutical manufacturing operations is critical not only for achieving productivity goals and safety, it also is a prerequiste for moving operations to a lean-manufacturing environment.

Interphex2007, New York, NY (Apr. 27)-The Interphex2007 Conference and Exhibition concluded here on Thursday. As the three-day event reached its conclusion, however, Interphex will continue its global push, with several shows scheduled abroad this year.

Interphex2007, New York, NY (Apr. 25)-One of the challenges facing pharmaceutical manufacturers is how to implement process analytical technology (PAT) into their commercial manufacturing processes. Michael Abad, engineering section manager, Abbott Laboratories shared insight from a project within Abbott in his presentation "Engineering for PAT" at Interphex today.

Interphex2007 (Apr. 25)-As the pharmaceutical industry moves to a risk-based approach in manufacturing, analytics will play a critical role in not only meeting regulatory requirements but also in building needed collaboration between product development and manufacturing groups.

Interphex2007, New York, NY (Apr. 25)-Interphex will see a new venue in 2008 and be co-located with a biotechnology exhibition. These are some of the changes in store for next year?s installment of the conference and exhibition, including a change in venue for next year?s conference.

Interphex2007, New York, NY (Apr. 24)-Effective project management is critical for successful outsourcing between a pharmaceutical company or sponsor and a contract manufacturing organization (CMO), and the strategies for such success was highlighted at the Interphex2007 Conference and Exhibition.

Interphex 2007 Conference and Exhibition, New York, NY (Apr. 24)-Interphex 2007 Conference and Exhibition opened today with nearly 1000 exhibiting companies and record attendance anticipated for the show.

Interphex, New York, NY (Apr. 24)-Compared with other industries such as the automotive industry, the pharmaceutical industry has been slow to adopt lean manufacturing. This situation, however, is changing.

Toronto (Apr. 17)-Patheon, Inc. plans to restructure its current network of six pharmaceutical manufacturing facilities in southern Ontario, Canada as part of its strategy to focus on manufacturing prescription pharmaceutical products and to improve its profitability.

Chalfont St. Giles, UK (Apr. 16)-GE Healthcare, a unit of General Electric, acquired Wave Biotech LLC, a supplier of disposable manufacturing technologies and processing equipment for the biopharmaceutical industry.

London (Mar. 29)-GlaxoSmithKline (GSK) plans to invest EUR 250 million ($334 million) at its production site at Currabinny, County Cork, Ireland over the next five years, according to a release issued by the Irish Development Agency (IDA Ireland, Dublin).

Washington, DC (Apr. 3)-The Synthetic Organic Chemical Manufacturers Association (SOCMA) adopted a policy on Inherently Safer Technology (IST) at its March meeting.

Singapore (Mar. 29)-The contract manufacturing organization Lonza Group (Basel, Switzerland) and Bio*One Capital (Singapore) broke ground for their large-scale commercial mammalian cell-culture manufacturing facility at Tuas Biomedical Park in Singapore.

Thousand Oaks, CA (Apr. 3)-Amgen, Inc. has provided a new schedule for the completion of a new manufacturing facility in County Cork, Ireland.