
Reflecting a strategic interest to strengthen its position in biologics, Bristol-Myers Squibb agreed to acquire the biopharmaceutical company Adnexus Therapeutics for $430 million.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Reflecting a strategic interest to strengthen its position in biologics, Bristol-Myers Squibb agreed to acquire the biopharmaceutical company Adnexus Therapeutics for $430 million.

Rep. John D. Dingell (D-MI), chairman of the Committee on Energy and Commerce, along with Reps. Frank Pallone (D-MI), chairman of the Subcommittee on Health, and Bart Stupak (D-MI), chairman of the Subcommittee on Oversight and Investigations, introduced legislation that would create a user fee on imported drug and food shipments.

The US Food and Drug Administration has denied shipments of active pharmaceutical ingredients manufactured at a production facility of Kunshan Chemical and Pharmaceutical Co. for violation of good manufacturing practices, according to an FDA warning letter issued Sept. 6, 2007.

Pfizer plans to cease all remaining manufacturing operations at its facility in Sandwich, Kent, United Kingdom. The closure will result in the loss of approximately 420 jobs, phased over the next two years, according to a company release

454 Life Sciences, part of Roche, and researchers at Columbia University identified a virus associated with the deaths of 2.4 million honey bee colonies.

The US Food and Drug Administration issued a final guidance, Manufacturing Biological Intermediates and Biological Drug Substances Using Spore-Forming Microorganisms, which provides recommendations that allow for greater flexibility when manufacturing biological products with spore-formers.

The US Food and Drug Administration issued a draft guidance, Pharmacogenomic Data Submissions Companion Guidance to be used as a companion to an earlier guidance, Pharmacogenomic Data Submissions, which was issued in March 2005.

Aptuit expands its drug-development capabilities with the formation of Aptuit Laurus to take advantage of the growing pharmaceutical outsourcing market in India.
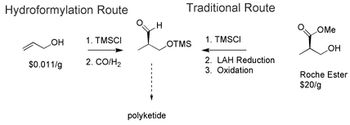
Researchers forward approaches for catalytic hydroformylation, asymmetric hydrogenation, and biocatalysis to achieve enantioselectivity.

Jason Kamm, managing consultant with Tunnell Consulting discusses the challenges and opportunities for pharmaceutical manufacturers in ICH Q10, the draft guidance on pharmaceutical quality systems issued by the International Conference on Harmonization.

The draft guidance ICH Q10 for pharmaceutical quality systems is part of the ongoing move to a science- and risk-based approach in manufacturing.

Identifying polymorphs is a crucial part of the drug-development process as researchers forward select methods to improve detection.

Contract manufacturers and pharmaceutical ingredient suppliers proceed with select investments in biologics manufacturing, small-molecule synthesis, and formulation as the industry prepares for CPhI Worldwide in Milan.

Pfizer (New York) proceeded with two agreements this week: a drug-development deal with Bristol-Myers Squibb (New York) and a license agreement with Xoma (Berkeley, CA) for antibody technology.

The US Food and Drug Administration is seeking public comments on a study designed to investigate the impact of visual distraction and the interplay of different sensory modalities (e.g., verbal, visual) used to present risk and benefit information during a television prescription drug advertisement.

The biopharmaceutical company Biopartners recently published data that showed its biosimilar "Valtropin" (somatropin), a recombinant human-growth hormone product, demonstrated the same safety and efficacy as Eli Lilly’s "Humatrope" in children with growth hormone deficiency.

Asia–Pacific is strongly positioning itself in the global pharmaceutical market, according to a recent analysis by PricewaterhouseCoopers.

GlaxoSmithKline reports that the US Department of Health and Human Services placed another order to purchase bulk H5N1 antigen for the US national stockpile of prepandemic vaccines.

A spate of drugs are scheduled to come off patent, offering vast potential and competition.

The financial performance of the pharmaceutical majors was generally favorable through the first half of 2007, with most companies reporting moderate to double-digit growth. Industry leader Pfizer, however, reported a sales decline for the second quarter and flat revenues through the first half of 2007. Pfizer Chairman and CEO Jeffrey Kindler says the company remains committed to its plans for cost-cutting, more outsourcing, and increasing its position in biologics.

The company begins production at a new $100-million manufacturing facility for prefilled injection systems, plans further investment in packaging facilities, and targets both early-phase development and commercial manufacture.
Catalytic routes to producing atorvastatin and sitagliptin are recent advancements.

Moheb Nasr, director of the Office of New Drug Quality Assessment in the Center for Drug Evaluation and Research at the US Food and Drug Administration, addressed the agency’s history and progress in moving to a science- and risk-based approach in regulation at the Pharmaceutical Technology Annual Conference.

Rapid microbial testing in biopharmaceutical manufacturing is an important tool in potentially reducing process risk and manufacturing costs due to loss of production material. Amy McDaniel, associate director of the QC Microbial Science and Technology Department with Wyeth Biotech, discussed the company’s evaluation and implementation of rapid microbial testing at Wyeth’s facility in Andover, Massachusetts, at the Pharmaceutical Technology Annual Conference.

Xcellerex, Inc. received two Phase-I contracts from the Defense Advanced Research Projects Agency (Arlington, VA) for the Accelerated Manufacture of Pharmaceuticals program.

Although North America accounts for the largest share of the pharmaceutical market, Brazil, China, India, Indonesia, Mexico, Russia, and Turkey are projected to account for almost one-fifth of the global market by 2020. The rising participation in select countries' drug-development activities is evident by recent investment and outsourcing by the pharmaceutical majors.

Abraxis BioScience, Inc. plans to separate its hospital-based product business, Abraxis Pharmaceutical Products (APP), from its proprietary products businesses, Abraxis Oncology and Abraxis Research, to form two public companies. Abraxis Pharmaceutical Products will become APP Inc., and Abraxis Oncology and Abraxis Research will become the new Abraxis BioScience.

Catalytic routes to producing atorvastatin and sitagliptin are recent advancements.

Double-digit growth is projected for the US generic drug market, and the industry positions for opportunities in biosimilars.

Pfizer CentreSource is proceeding with a new plan for the supply of steroids and steroid intermediates through a recently signed pact with two Asian contract manufacturing organizations.