
How will the move to more specialized and personalized medicines impact drug delivery and dosage forms?
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

How will the move to more specialized and personalized medicines impact drug delivery and dosage forms?

Emerging markets are an engine for growth in the custom-manufacturing and pharma markets.

Bristol-Myers Squibb agrees to sell global diabetes business to AstraZeneca for $2.7 billion upfront plus an addition $1.4 billion in potential regulatory- and sales-based milestone payments.

AstraZeneca issues a statement regarding the launch of esomeprazole strontium in the United States.

Biogen and Samsung Bioepis, a joint venture between Samsung Biologics and Biogen Idec, advance plans for anti-TNF biosimilar product candidates.

Pfizer settles its litigation against Teva Pharmaceuticals regarding Pfizer's patent covering the use of Viagra.

GlaxoSmithKline plans to expand its facilities in Ware and Worthing in the United Kingdom and will build a new facility for manufacturing innovation.

Teva Pharmaceutical Industries releases a financial outlook for 2014 based on two possible scenarios concerning its multiple-sclerosis drug Copaxone (glatiramer acetate).

Pharmaceutical Technology takes a look at the major deals and moves of 2013.

Global spending on medicines is expected to meet the $1 trillion threshold in 2014 and reach $1.17 trillion by 2017, according to a recent report by the IMS Institute for Healthcare Informatics
Fine-chemical companies, contract manufacturers, and researchers advance chemocatalysis and biocatalysis.
A review of this year's crop of the new molecular entities and new biologics license applications approved by FDA thus far in 2013.

Annual growth in spending for medicines is expected to rise from 2-3% in 2013 to 5-7% in 2017, the highest pace of growth since 2009.

GlaxoSmithKline will build a new solid dosage manufacturing facility in India.

Progress in delivery science, manufacturing technologies, and commercialization are playing critical roles in advancing the development of complex parenteral drug formulations for new drug substances having a variety of formulation challenges

Former President Jimmy Carter and Pfizer mark milestone for long-standing partnership to combat trachoma in the developing world.

Overall growth in the market for pharmaceutical contract research and manufacturing is strong although growth in established markets erodes at the expense of emerging markets.

A roundup of expansion activity of manufacturing capacity and service offerings from fine-chemical producers, CDMOs, and CMOs.
Fine-chemical companies, contract manufacturers, and researchers advance chemocatalysis and biocatalysis.

A US court of appeals rules on patent litigation for AstraZeneca's asthma drug Pulmicort Respules (budesonide).

As the pharmaceutical industry prepares for changes to compendial and regulatory standards for elemental impurity analysis, QA/QC and laboratory scientists are tasked with adapting their operations to include data management, analysis, and reporting based on inductively coupled plasma–mass spectrometry (ICP–MS).

Regulatory compliance is of paramount importance to pharmaceutical and biopharmaceutical companies, which must be continually prepared for inspections by FDA, EMA, and other health authorities to meet requirements for good clinical practices (GCP) and good manufacturing practices (GMP).

Formulation scientists tackle the challenges of improving drug absorption across the gastrointestinal membrane.

Overall growth in the market for pharmaceutical contract research and manufacturing is strong although growth in established markets erodes at the expense of emerging markets.

The adoption of quality by design in small-molecule drug development and manufacturing continues to evolve as the industry seeks ways to augment process understanding for APIs.

Brian Scanlan, CEO and president of Cambridge Major Laboratories, discusses the rationale behind the company's merger with AAIPharma Services.

Roche's plans for a major biologics manufacturing expansion is the latest investment by Big Pharma

AstraZeneca and SAFC are the latest companies to expand positions in antibody drug conjugates (ADCs).

Global biotechnology financing through the first nine months of 2013 shows that initial public offerings are on the rise while venture-capital funding stays relatively flat.

A recent market analysis offers a promising outlook for contract fill-finish and lyophilization services.

Published: January 9th 2013 | Updated:
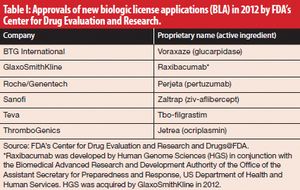
Published: February 2nd 2013 | Updated:

Published: February 1st 2013 | Updated:

Published: February 1st 2013 | Updated:

Published: January 9th 2013 | Updated:

Published: February 1st 2013 | Updated: