
Bristol-Myers Squibb outlined a comprehensive review of its operations and a strategy to improve top-line growth, a plan that involves staff reductions and rationalization of its manufacturing plants.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

Bristol-Myers Squibb outlined a comprehensive review of its operations and a strategy to improve top-line growth, a plan that involves staff reductions and rationalization of its manufacturing plants.
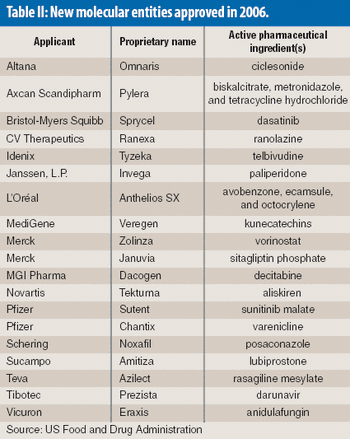
The number of new molecular entities approved by the US Food and Drug Administration is down this year as the pharmaeutical industry continues to face an innovation drought.

Big Pharma's strategic push into biologics and continuing restructuring are among the highlights for 2007.

Chi-wan Chen, deputy director of the Office of New Drug Quality Assessment (ONDQA) at the US Food and Drug Administration's Center for Drug Evaluation and Research, shares insight from FDA's pilot program that was designed to allow pharmaceutical companies to submit CMC information demonstrating application of quality by design.

A surge in capacity in contract microbial and mammalian cell-culture is underway to meet rising production needs for biopharmaceuticals.

GlaxoSmithKline agreed to acquire Reliant Pharmaceuticals for $1.65 billion in cash.

Eli Lilly plans to invest $100 million in research and development in China over the next five years through Lilly Asian Ventures, a Lilly-owned venture capital firm that makes investments in Asia.

Growth in the global pharmaceutical industry is expected to slow in 2008, marked by slowing growth in the United States and other major markets. At the same time, pharmaceutical industry growth in emerging markets is expected to reach double-digits, although these markets still represent a small percentage of global pharmaceutical sales.

The US Government Accountability Office issued findings that are largely critical of the US Food and Drug Administration in managing its inspections of foreign drug-manufacturing facilities.

The Interagency Working Group on Import Safety issued its action plan for ensuring the safety of imported products, including drugs, food, and consumer products, into the United States.

The Bulk Pharmaceuticals Task Force, an affiliate of the Synthetic Organic Chemical Manufacturers Association (SOCMA) weighed in on the Congressional debate concerning the US Food and Drug Administration?s inspection process of foreign drug- manufacturing facilities.

Looking to resolve litigation over Vioxx (rofecoxib), Merck & Co reached an agreement to settle the vast majority of Vioxx claims for $4.85 billion.

US Food and Drug Administration Commissioner Andrew C. von Eschenbach testified before Congress last week to outline the agency’s inspection process for foreign drug manufacturers and efforts to improve the agency’s information technology systems.

The US Food and Drug Administration’s effectiveness in regulating the manufacture of pharmaceutical products and active pharmaceutical ingredients at foreign facilities was questioned at a Congressional hearing last week. Congress, industry, and government officials weighed in on the issue.

Recent activity among contract manufacturing organizations include expansions for Lonza and Codexis in Asia and the addition of aseptic cytotoxic capabilities for DSM. Also, Vetter adds anticounterfeiting capabilities for prefilled syringes.

Ash Stevens is adding cryogenic capabilities as part of an overall strategy to focus on small molecules requiring smaller volumes and complex chemistry.

Equipped with a new sourcing strategy in Asia and improving market conditions, Pfizer CentreSource expects a good performance from its steroid business this year and in 2008.

Market demand for cytotoxic drugs is leading CMOs to expand their API manufacturing and formulation services.

Pfizer decided to stop investing in ?Exubera? (inhaled insulin), a product that had been considered a potential blockbuster drug.

GlaxoSmithKline and Synta Pharmaceuticals agreed to jointly develop and commercialize STA-4783. The drug is an injectable, small-molecule, oxidative stress inducer for treating metastatic melanoma that is entering Phase III clinical development.

CPhI Worldwide, the large trade show of suppliers of active pharmaceutical ingredients (APIs), intermediates, and excipients, took on a decidedly international presence.

The European Fine Chemicals Group (EFCG) issued a position paper on excipients used in pharmaceutical manufacturing at CPhI Worldwide last week.

The public gives mixed reviews to the pharmaceutical industry and the US Food and Drug Administration for issues such as drug safety, timeliness in bringing drugs to market, and their role in addressing the high costs of drugs.

Identifying polymorphs is a crucial part of the drug-development process as researchers forward select methods to improve detection.

The rising influence of Asia in the global pharmaceutical ingredients market was evident at CPhI Worldwide.

Restructuring among contract manufacturing organizations and suppliers of pharmaceutical ingredients this year is bringing a new group of faces to CPhI Worldwide this year.

A roundup of news from exhibitors at CPhI Worldwide.

Orally disintegrating tablets (ODTs) continue to attract attention as an alternative to conventional oral dosage forms.
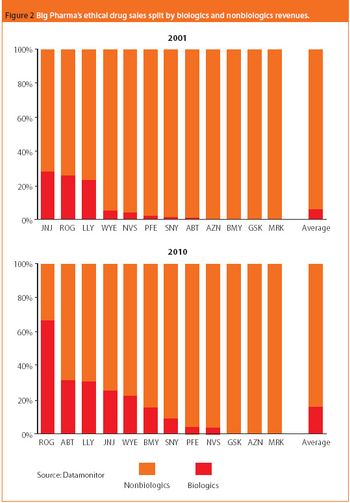
In 2007, the global pharmaceutical market is expected to grow moderately while biologics, generic drugs, and specialty-initiated drugs are projected to increase at double-digit rates. These trends for finished pharmaceuticals are reflected in the global market for APIs, where the merchant generic API market is expected to see strong demand. On a production basis, India and China are forecast to raise their shares of the global generic API market against industry strongholds Italy and Spain. Meanwhile, the US is expected to hold its its position as the leading producer of biotechnology-based APIs in an area traditionally dominated by captive production. And biogenerics or biosimilars gradually reshape the market.

The European Medicines Agency?s Committee for Medicinal Products for Human Use recommended the lifting of the suspension of the marketing authorization for ?Viracept? (nelfinavir mesylate) from Roche and the re-introduction of the drug in the European Union.