
Precedents set in the historic Barr case continue to raise questions over suitable sample-size criteria.

Precedents set in the historic Barr case continue to raise questions over suitable sample-size criteria.

Political leaders need to consider the impact of the biopharmaceutical industry on the economy.

Packaging is indeed headed to be a lead sector in the Asian pharmaceutical environment, but certain challenges must first be overcome.

A Q&A with Deborah Tanner, executive vice-president and group president of R&D laboratories at Covance, on recent industry trends.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.

Employee training-at all levels-is crucial for moving forward with a successful risk- and quality-based manufacturing strategy.

The last in a series of eight case studies from the Product Quality Research Institute focuses on internal GMP audits.
The sixth in a series of eight case studies from the Product Quality Research Institute focuses on packaging line GMP optimization.

EMA Hosts Subgroup Analysis Workshop.

On Nov. 19, 2011, Ben Venue Laboratories voluntarily and temporarily suspended the manufacture and distribution of products made at its Bedford, Ohio, facility. These products include Doxil (doxorubicin HCl liposome injection), which the company produces for Johnson & Johnson. The company's clients include Pfizer, Hospira, and Teva.

FDA Holds Special Press Briefing Regarding Revocation of Genentech's Avastin.

ICH Steering Committee Approves Revised Guideline for Genotoxicity Testing and Data Interpretation for Medicines.

Until now, the industry has adhered to the tradition of producing three batches of product to validate its manufacturing processes. But FDA?s new process-validation guidance does not prescribe any number of batches that is necessary for compliance.

EMA Issues Concept Paper for Public Consultation on Development of Toxicological Guidance for use in Risk Identification.

Clamor mounts over compromised care and rising costs due to lack of crucial therapies.

Careful mixing during a product's distillation can help avert trouble from a strong concoction.

Novartis AG and Novartis Pharmaceuticals Corporation have been focusing research efforts on rare diseases since the company was established in 1996.
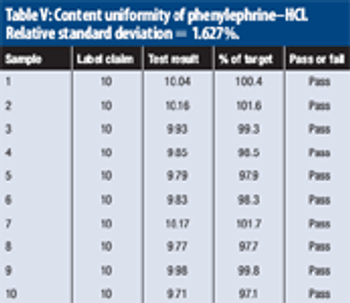
The study evaluates near-infrared analysis of tablets nominally containing 4 mg of chlorpheniramine maleate and 10 mg of phenylephrine hydrochloride per dose.
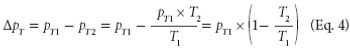
The authors describe the operational qualification of test accuracy with regard to temperature drift using a thermal-compensation algorithm on several freeze dryers.
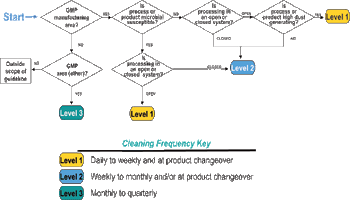
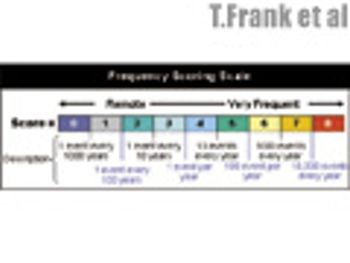
The fifth in a series of eight case studies from the Product Quality Research Institute focuses on nonsterile facility cleaning requirements.

Russia is aiming to provide an alternative to China and India for drug manufacturing, including APIs.

The authors propose a plan to keep the US pharma industry afloat and in the lead.
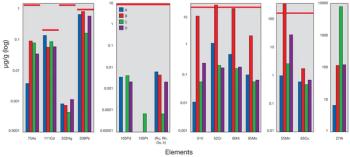
The US Pharmacopeia (USP) proposes to lower the maximum permissible limits of trace elements in pharmaceuticals and recommends that impurities be measured through automated instrumentation-based methods. The proposed regulations specify inductively coupled plasma–mass spectrometry (ICP–MS) and inductively coupled plasma–optical emission spectrometry (ICP–OES) as the techniques of choice. This article discusses the benefits of ICP–MS and ICP–OES for the accurate detection of trace elements in pharmaceutical products, in compliance with the proposed USP chapters.

June and July saw three major US conferences on implementing single-use technologies: the IBC Single-use Applications meeting, the PDA Single-use Workshop and the Bio-Process Systems Alliance (BPSA) International Single-use Summit (ISUS). Jerold Martin highlights some of the key topics discussed at these meetings.
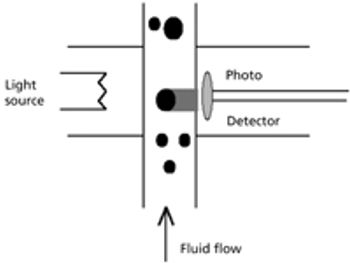
This article focuses on the history of glass delamination and methods that detect it, both from a compendial and a research perspective.