
The author outlines the scientific aspects of forced degradation studies that should be considered in relation to ANDA submissions.

The author outlines the scientific aspects of forced degradation studies that should be considered in relation to ANDA submissions.

Apple's experience with manufacturing facilities in China present opportunity for future best practice.

How niche strategies can offer mainstream potential for biopharmaceutical companies.

Poland's government aims to make the Eastern European country a biotech powerhouse.

Q&A with David Elder of Strategic Compliance Consulting, PAREXEL International on responding to a 483 within 15 days. Elder is a former senior official with FDA.
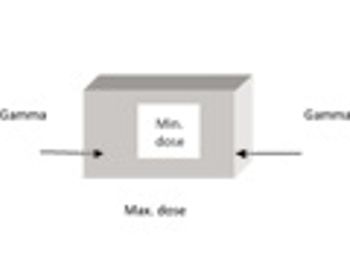
This paper examines the process of gamma irradiation of plastic materials used as part of single-use disposable systems in the pharmaceutical and biotechnology sectors, with a focus on validation requirements.

Parametric release and real-time testing use manufacturing data to ensure that products are made according to defined standards. PharmTech talks to Boehringer Ingelheim's Heribert Hausler about these issues.

The authors provide a review of test methodology and standards, including current industry and regulatory proposals, for biological indicator growout times.

FDA has issued Warning Letters to 10 companies that manufacture and distribute dietary supplements that contain dimethylamylamine. FDA cited the companies for marketing the supplements without submitting evidence that the products are safe.
Readers share their views on bioprocessing challenges, equipment use, and outsourcing trends in our annual bioprocessing equipment and processing survey.

FDA has released a final guidance describing cGMP for preparing media fills for validation of aseptic preparations for positron emission tomography drugs.

The management board of the European Medicines Agency has introduced a range of new measures to strengthen and extend its conflicts of interest policy for scientific-committee members and experts, as well as for members of the management board.

The decision by the Indian patent authorities to issue a compulsory license for a generic version of a Bayer anticancer drug engenders debate.

Collaboration can begin with a conversation.

Failure to disclose info may work sometimes, but eventually every question will be answered.

The importance of new drug trials to patients, the economy, and science.

Understanding the differences between convenience, target, and self-selected samples.
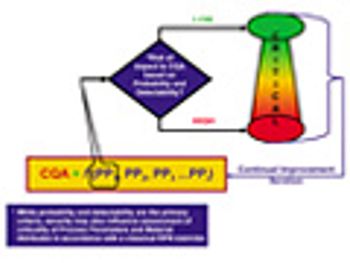
The authors provide an overview of the new ISPE Guide Series on Product Quality Lifecycle Implementation and how the guides can be used in a complementary way with existing guidance from FDA and the International Conference on Harmonization.

A Q&A with Erik van den Berg, CEO of AM-Pharma, on recent industry trends.

Soaring opioid use creates challenges for new drug development and supply-chain control.

Comparison of the top GMP deficiencies cited by the PIC/S Participating Authorities.

The confluence of science, technology, and regulation can provide path forward.

China's drug-distribution network has been a mess for years, but government reforms and industry focus are unveiling new opportunities for market order and growth.

The contract provider needs to know as much as the NDA holder.

The authors detail the possible consequences of noncompliance and a lack of quality control.