
The ‘full tolerance coverage method’ is introduced as a coverage estimation approach for assessing the uniformity of dosage units from large sample sizes, ensuring that no dosage unit exceeds the specification range.
Pramote Cholayudth previously was the managing director of the Professional Conference Center, Ltd. He currently is a validation consultant to Biolab Co., Ltd., in Thailand. He is the founder and manager of PM consult, cpramote2000@yahoo.com.

The ‘full tolerance coverage method’ is introduced as a coverage estimation approach for assessing the uniformity of dosage units from large sample sizes, ensuring that no dosage unit exceeds the specification range.

This article introduces that the basic technique for tolerance interval is implemented using the conventional range 85–115% LC where the proposed range in the PF is not taken into account.

This article discusses the evaluation of paired content uniformity and weight variation data sets using F and T tests to ensure that batches meet compendial requirements.

This article introduces a control chart technique using F statistic for continued process verification of oral solid dosage forms.

This article is focused on introducing a control chart technique using relative standard deviation (RSD) statistics (i.e., RSD chart); in other words, a coefficient of variation chart for continued process verification.
The author introduces a practical approach to determining the best estimate of probability for passing multiple stage dissolution tests.

This article introduces a practical approach to determining the best estimate for probability of passing uniformity of dosage units (content uniformity) using probability vs. lot coverage charts and tables constructed using simulated probability and lot coverage (LC1) data.

The CuDAL-Excel program, based on Microsoft (MS) Excel, has been developed to calculate the United States Pharmacopeia (USP) passing probability of content uniformity and dissolution tests for both sampling plan 1 and sampling plan 2 scenarios and for both immediate release and extended release requirements. The users can obtain the passing probability by simply entering the input variables, with wide applications for process validation/verification and batch release. As a user-friendly program, CuDAL‑Excel should bring more benefits to the industry practitioners than other existing programs/tools.

This paper describes how the concept of acceptance value can be redefined to remove bias and more closely reflect quality targets.

The working acceptance limits for acceptance values (AV) are determined using the critical values at, for example, 95% coverage over the corresponding AV distributions. However, validity of such limits needs to be elaborated.

The author describes how to establish acceptance limits for acceptance value (AV) data for process validation batches, typical characteristics of AV distributions, and how to derive relevant constants for AV control charts in annual product review and continued process verification reports.
This article introduces the concepts of pooled variance and the central limit theorem, which are intended for establishing acceptance criteria for blend uniformity data of granular powder blends when a significant degree of sampling bias is involved.

The Uniformity of Dosage Units test is used to evaluate content uniformity or weight variation or dosage forms such as tablets and capsules. The author introduces two different acceptance value limits (n = 10 and 30) in this article.
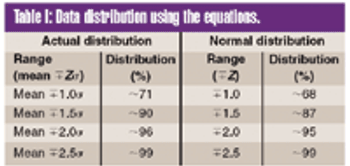
To meet the requirements of the USP ?755? Minimum Fill and ?698? Deliverable Volume tests, target fill levels greater than 100% must be established. This article proposes a criterion for establishing an appropriate target fill level such that a sample will have a 95% probability of passing these USP tests at 95% confidence.

A straightforward approach reveals how the probability of passing the USP content uniformity test can be calculated for tablets and capsules.

Published: March 3rd 2023 | Updated:

Published: March 10th 2025 | Updated:

Published: February 3rd 2024 | Updated:

Published: July 3rd 2022 | Updated:

Published: October 3rd 2022 | Updated:
Published: May 3rd 2021 | Updated: