
Sharp Packaging Services adds Biotechnology Center of Excellence to its to its Allentown, PA campus.

Sharp Packaging Services adds Biotechnology Center of Excellence to its to its Allentown, PA campus.
Award-winning drug packaging innovations include interactive cartons and multifunctional labels.

The company sees a demand for customized individual packaging solutions as the pharmaceutical manufacturing industry moves towards smaller lot sizes that require aspects of individualization.

The Gx RTF ClearJect syringe is made of cyclic olefin copolymer and is tungsten- and adhesive-free.

The Novelia system has been approved as a packaging component and delivery system of multidose drug product formulated without preservatives.

If FDA’s proposed generic-drug labeling rule is passed in 2017, generic drug companies would need documented processes for safety tracking and label updates.

Visual inspection of parenteral vials is the first step in a root cause investigation.
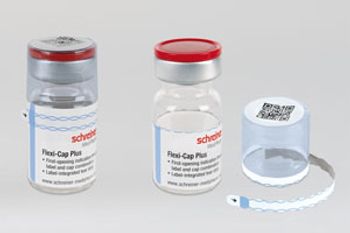
Schreiner MediPharm’s Flexi-Cap Plus incorporates a label-integrated tear strip for enhanced security.

The company is voluntarily recalling one lot of product due to a potential packaging mistake.

Arbor Pharmaceuticals is voluntarily recalling Cetylev (acetylcysteine) effervescent tablets due to inadequate seal of the blister pack.

TOPAS Advanced Polymers announces its COC materials are compliant with new USP standard for pharma plastic packaging systems.

Innovations protect product quality, improve productivity, enhance sustainability, and simplify usage for patients and caregivers.

Herma’s wrap-around, high-speed labelling machine will be available for demonstration at its new facility in New Jersey.

GenPak Solutions is cited by FDA for cGMP violations at its Hilliard, Ohio facility.


Innovations protect product quality, improve productivity, enhance sustainability, and simplify usage for patients and caregivers.
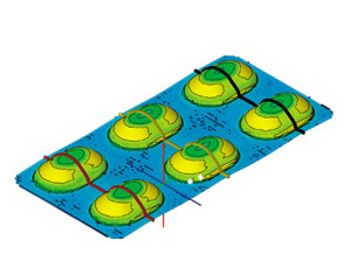
Science-based software and testing services expedite the material selection process and ensure blister packs deliver adequate barrier protection for solid-dosage forms without over-packaging.

Packaging technologies for serialization, parenteral products, and solid-dosage forms were displayed at INTERPHEX 2016.

The agency cited Apotheca Supply, a repackager and relabeler of pharmaceuticals for CGMP violations.

Packaging innovations displayed at INTERPHEX include advanced materials, inspection systems, and filling systems.

INTERPHEX exhibitors will display software, printing, and packing equipment designed for serialization.

The FDA guidance describes information to provide to the agency when submitting a proposed proprietary name.

Non-destructive container closure integrity testing allows 100% inspection of all ampoules, syringes, vials, and cartridges.

Consider the 3 Ps (product, process, and patient) when choosing a parenteral packaging material.

Science-based software and testing services expedite the material selection process and ensure blister packs deliver adequate barrier protection for solid-dosage forms without over-packaging.