
Was FDA's decision to issue a draft guidance on of-label information, amidst Congressional scrutiny, the right thing to do?

Was FDA's decision to issue a draft guidance on of-label information, amidst Congressional scrutiny, the right thing to do?

The annual show provided one-stop shopping for packaging equipment and materials.

Companies continue to develop inhaled insulin and other drugs, despite the problems that Pfizer's "Exubera" experienced.
Manufacturers expect to see the latest developments in process equipment at INTERPHEX, and this year's show was no disappointment. Exhibitors regularly display additions to their lines of encapsulators, tablet presses, material-handling machines, and other automated manufacturing equipment. But the products on view in Philadelphia Mar. 26–28 were not limited to manufacturing applications.

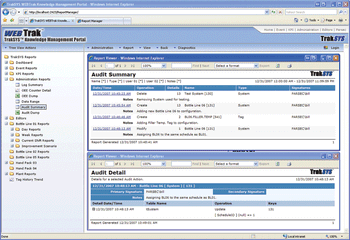
Undertaking process validation involves a major commitment in terms of personnel, resources, time, and money. Performing prerequisite verifications can reduce the risk of making costly mistakes. This Part 1 article explains the value of performing prerequisite verifications and presents case-study examples and real-world solutions to avoid costly process validation failures.

What makes a drug ripe for respiratory delivery?

Heparin contamination casts a shadow on regulatory oversight of product quality.

Brief pharmaceutical news items for April 2008.
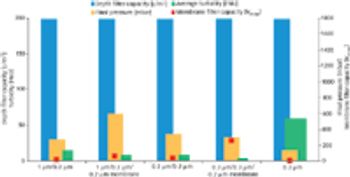
Monoclonal antibodies and recombinant proteins have increased their importance and gained success as therapeutic agents in the treatment of various diseases.
Biopharmaceuticals are the most rapidly growing segment of the pharmaceuticals market. Developing and marketing biopharmaceuticals are huge roles in almost every major pharmaceutical company's strategy. However, they are extremely complex molecules and are highly sensitive to the manufacturing processes used to produce them. These processes require exquisite control of living production systems, making, without a doubt, biopharmaceuticals one of the most challenging products of any type to manufacture.


Manufacturing facilities must be inspected by members of regulatory bodies. However… these bodies are woefully inadequate at performing the task.

The amount of water used by industry, including pharma and biotech manufacturing, amounts to 23% of the world's supplies
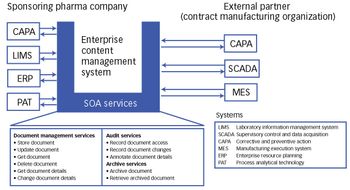
As a skipping stone creates ripples in a lake, SOA can help create benefits that quickly ripple through many other areas of the organization and partners.

A microelectronic system based on radio-frequency (RF) cell ablation addresses limitations of other transdermal drug-delivery methods. This system expands the transdermal spectrum to include the delivery of water-soluble molecules, peptides, proteins, and other macromolecules.

Liquid and semisolid encapsulation using two-piece hard capsules is an ideal drug delivery approach for highly potent compounds and poorly water-soluble drugs. The authors detail the factors to reduce risk when designing and operating a facility for secondary manufacturing of highly potent drugs.

US Representatives Anna G. Eshoo (D-CA) and Joe Barton (R-TX) introduced legislation this month week to create a regulatory pathway for biosimilars, or follow-on biologics.

In a press release, the University of Texas announced that Mauro Ferrari, of the University?s Health Science Center at Houston, presented a proof-of-concept study of a new multistage delivery system for imaging and therapeutic applications.

Also, Alkermes announces restructuring and reduction of workforce, Icagen announces several senior management promotions, more...

Scientists at the University of North Carolina at Chapel Hill have successfully tested a new inhaled tuberculosis (TB) vaccine.

Sandoz introduced its "Omnitrope Pen 5" with liquid cartridge in the United States. The product was approved by the US Food and Drug Administration and is a new form of the first follow-on version of a recombinant biotechnology drug.

As part of ongoing efforts to improve the quality of imported drug products, the US Food and Drug Administration is setting up shop in China.

Also, Pipex Pharmaceuticals implements cost-cutting measures, Pfizer's Senior Vice-President and General Counsel Allen Waxman leaves the company, more...

Hoping to provide a better understanding of its role in the heparin contamination investigations, the US Food and Drug Administration has posted a series of information sheets on its website.

This issue of Equipment & Processing Report features products from Eriez Magnetics and Netzsch Fine Particle Technology