
Also, PDL BioPharma will no longer pursue sale of the company, executives resign from Topigen Pharmaceuticals, more...

Also, PDL BioPharma will no longer pursue sale of the company, executives resign from Topigen Pharmaceuticals, more...

Eli Lilly terminated the development of its inhaled insulin product AIR, a diabetes treatment that had been in Phase III clinical trials.

The 59th Pittsburgh Conference gathered more than 1000 exhibitors on its showroom floor this week.

Also, Millipore plans to open Singapore facility, Michael J. Simms joins Alexza Pharmaceuticals, more...

With another potential "made-in-China" crisis looming over the recall of heparin, critics and the media seem to be waiting in line to take another jab at the US Food and Drug Administration.

Draft federal legislation that would require high-risk chemical facilities to use inherently safer technology for reducing their risk may present potential problems for custom and batch manufacturers supplying the pharmaceutical industry.

Quality-by-design submissions may reduce supplements and improve change management.

After two centuries, there's no reason to maintain two tablet compression tooling standards.

A. Nair discusses patent disputes in India.

If not properly monitored, filters and plastic bags can keep back more than they should.

New packaging options monitor and protect temperature-sensitive products.
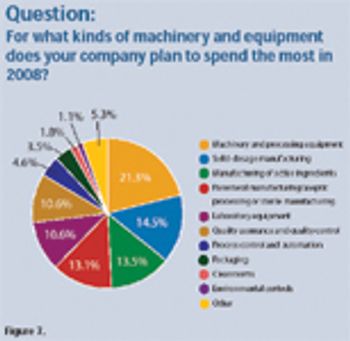
The pharmaceutical industry plans moderate increases in spending for equipment and machinery in 2008. Investments include equipment for solid-dosage manufacturing, active pharmaceutical ingredients, and parenteral manufacturing.
New research and ideas for March 2008

Contract manufacturers of APIs and intermediates are cautiously optimistic.

Chemical imaging of solid dosage forms has become a powerful analytical tool for the development of solid dosage forms.

The selection of an appropriate salt form for a potential drug candidate is an opportunity to modulate its characteristics to improve bioavailability, stability, manufacturability, and patient compliance.

Editors' Picks of Pharmaceutical Science & Technology Innovations; Analytical system provides multiuser capability; Encapsulator aids dosage design; Versatile drive offers quick setup

Show blasts off this month in Philadelphia with more suppliers, new trends, and real-world solutions.
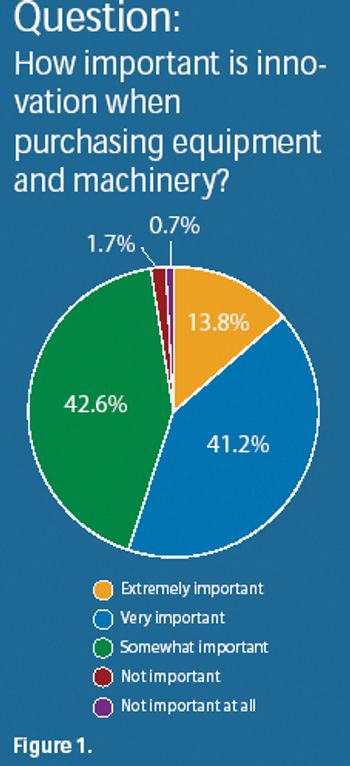
Results from Pharmaceutical Technology's Equipment and Machinery Trends survey and industry members provide insight into product innovation

A preview of some product enhancements and launches for Interphex 2008, the large trade show being held Mar. 26–28 in Philadelphia.
A news roundup for March 2008.

In this topical review, the authors discuss the rationale behind microstructural requirements for biopharmaceutical equipment and problems that may be encountered during the fabrication of high-performance corrosion-resistant equipment.

Brief pharmaceutical news items for March 2008.
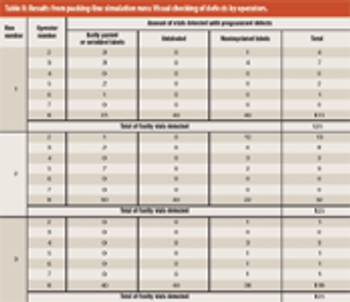
This article focuses on upgrading and improving a packing process to comply with current good manufacturing practices. The authors sought to maintain proper quality assurance for finished products.

Twenty years ago it was commonplace for pills, tablets and capsules to come in small, plastic or even glass bottles. Syrups were a much more common galenic solution than today, and individual dosages of injectables were only offered in glass vials and ampoules.