
On Mar. 29, 2012, the Generic Pharmaceutical Association reiterated its call for Congress to move forward with user-fee proposals for generic drugs and biosimilar products.

On Mar. 29, 2012, the Generic Pharmaceutical Association reiterated its call for Congress to move forward with user-fee proposals for generic drugs and biosimilar products.

With around 80% of APIs manufactured outside the US, many in developing nations without strong regulatory oversight.

Pharmaceutical Technology's annual survey on equipment and machinery reveals the spending levels and type of spending made in 2011 and planned for 2012.
In Part III of a three-part article, the authors examine various degradation routes of APIs, impurities arising from interactions in formulations, metabolite impurities, various analytical methods to measure impuritie, and ways to control impurities.

Critical issues that should be considered when scaling up a hot-melt extrusion process.

Collaboration can begin with a conversation.

With financing constrained, biotechnology firms must find ways to sustain innovation.

Contract API manufacturers and fine-chemical producers roll out capacity and service expansions.

How to use geographic diversification and legacy technology transfers to avoid product shortages.

Visitors will see many packaging innovations at the annual industry exhibition.

Failure to disclose info may work sometimes, but eventually every question will be answered.
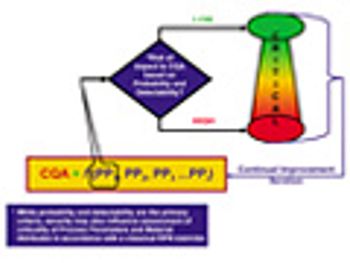
The authors provide an overview of the new ISPE Guide Series on Product Quality Lifecycle Implementation and how the guides can be used in a complementary way with existing guidance from FDA and the International Conference on Harmonization.

A Q&A with Erik van den Berg, CEO of AM-Pharma, on recent industry trends.

Experts discuss the best practices for developing a QbD-based lyophilization process.

Soaring opioid use creates challenges for new drug development and supply-chain control.

China's drug-distribution network has been a mess for years, but government reforms and industry focus are unveiling new opportunities for market order and growth.

The contract provider needs to know as much as the NDA holder.

Greece’s economic crisis has battered the country’s healthcare system, resulting in medicine shortages, market withdrawals and falling profits for the pharma industry.

There are no two completely identical freeze dryer units in operation anywhere.

Lyophilisation is often necessary for pharmaceutical products to improve stability or shelf-life. However, the process can present difficulties, particularly when scaling up from the laboratory to commercial production. We bring experts together to discuss best practices for developing a lyophilisation process, including quality by design (QbD) and design space.

Modular factory concepts, which take flexible manufacturing to the next level, are beginning to take hold in the biopharmaceutical industry.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the March 2012 edition from EMD Millipore, ARTMiS, and Powder Systems.
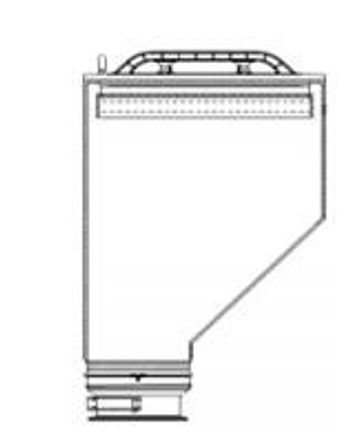
Powder-container design affects discharge rate, cleanability, and powder flow. Flow problems, such as ratholing, arching, and erratic flow, can be eliminated with appropriate container geometry.

In our tablet coating process, we are losing up to 15% of our coating solution in each processing run. What can we do to prevent this problem in order to reduce waste and increase our cost efficiency?

“One of the most daunting challenges facing pharmaceutical companies is securing the long and complex supply chains that are typical in today’s global industry.