
US Pharmacopeia documents best supply-chain practices and seeks broad input on proposal.

US Pharmacopeia documents best supply-chain practices and seeks broad input on proposal.

In Part II of a three-part article, the authors examine impurities from chiral molecules, polymorphic contaminants, and genotoxic impurities.

Has the long-awaited guidance answered all of the industry's questions?

Social media use raises questions about applying old standards to new information technology.

New product reviews for March 2012.

The evolving bio/pharmaceutical business model poses risk for CMOs.

A Q&A with Chris Meissner, president of Meissner Filtration Products, on recent industry trends.

The author reviews significant changes to GMP for excipients in the forthcoming American National Standard, including a risk-based approach to excipient manufacture, why new requirements were proposed, and their potential impact to excipient manufacturers.

The article examines some recent developments for this process step and for continuous manufacturing overall.

This article provides guidance for industry on how to comply with the pending American National Standard on excipient GMP, with a focus on risk assessment.
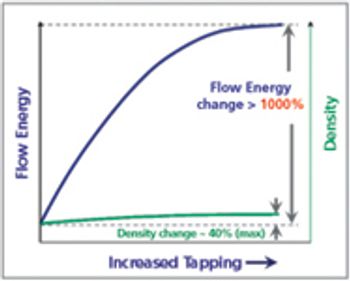
This article considers the different conditions to which the powder is subjected in the tableting process, and discusses which powder properties should be measured to accurately reflect likely powder behavior in the process.

Experts in solid dosage discuss the formulation and manufacture of multilayer tablets.

Traditionally more prevalent in less industrialised regions, counterfeit medicines are now more frequently entering the heavily regulated supply chains of EU countries.

Boehringer Ingelheim's Heribert Häusler tells us about parametric release and real-time testing.

Regulatory bodies, standard-setting organizations, and industry seek to tackle the problem of counterfeit drugs and securing the flow of pharma ingredients.
The authors describe a new assembly for bulk and final drug product filling operations.

With the increasing use of hot-melt extrusion, extruder manufacturers have developed mid-size twin-screw extruders that facilitate process development and scale-up to commercial processes.
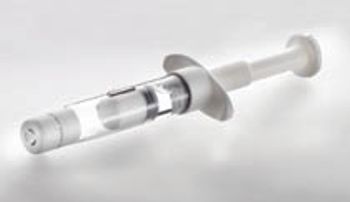
The author discusses the relative advantages and disadvantages of lyophilization in vials and dual-chamber systems.

We need to measure the particle size of a moisture-sensitive powder. We would like to use dry analysis on our laser-diffraction system, but the particles are fragile and we're struggling to get robust results. Is wet measurement with a nonaqueous solvent the only option?

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the February 2012 edition from ACS Valves and G-Con.

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

Pharma announces plans for the year ahead at annual JPMorgan Global Healthcare conference.

A nickel's worth of free advice to the competition could come at the expense of your bottom line.

In Part I of a three-part article, the authors discuss what constitutes an impurity and the potential sources of impurities in APIs and finished drug products.
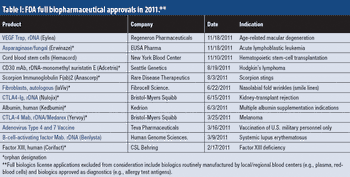
Industry optimism is on the rise for 2012.