
To meet the demands of early-stage development, contract research organizations can evaluate various dosage-form options. The author examines various methods of capsule filling, including binary blends.

To meet the demands of early-stage development, contract research organizations can evaluate various dosage-form options. The author examines various methods of capsule filling, including binary blends.
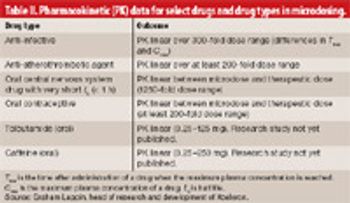
J. Scott Tarrant, executive vice-president of Xceleron, explains the role of microdosing in drug development. He describes how microdose data can be used to predict pharmacological dose absorption, distribution, metabolism, and excretion/pharmacokinetic outcomes using accelerator mass spectrometry.

Also, Cobra Biomanufacturing to extend collaboration and form joint venture with ViroMed, Epix Pharmaceuticals CEO resigns, more...

Pharmaceutical companies developing new drug candidates for Hepatitis C virus infection now can test their compounds with a novel culture system that mimics the biology of HCV infection in humans.

Also, Niro changes its name to GEA Process Engineering; Frances K. Heller joins Exelixis as executive vice-president of business development, more...

3M Drug Delivery Systems has successfully designed a proof of concept device using a solid microstructured transdermal system for the systemic delivery of high-potency pharmaceuticals. The technology was showcased at a poster session at the annual meeting of the Controlled Release Society held this week in New York City.

Also, Roche to end HIV/AIDS research program, WuXi PharmaTech makes appointments, more...

Also, Catalent Pharma Solutions to collaborate with One World Design and Manufacturing Group, Bioheart appoints Howard J. Leonhardt as CEO, more...

The European Pharmacopeia Commission has published the General Information chapter "Potentially Genotoxic Impurities and European Pharmacopoeia Monographs on Substances for Human Use" in the July 2008 edition of PharmEuropa.

Also, Covance and WuXi PharmaTech to form contract research joint venture in China, Covidien makes appointments to its Pharmaceutical Products and Imaging Solutions businesses, more...

Molecular Profiles created a poster that demonstrates the efficacy of its "nanoPASS" (nanoscale predictive analytical screening solution) technique to characterize an active pharmaceutical ingredient's (API) surface energy.

The Congressional Budget Office has released a report that provides a picture of the financial impact from the enaction of S.1695, the Biologics Price Competition and Innovation Act of 2007.
Drugmakers seeking to block the activity of a protein may have a new strategy at their disposal.

This review article explains how self-emulsifying drug delivery systems can increase the solubility and bioavailability of poorly soluble drug.
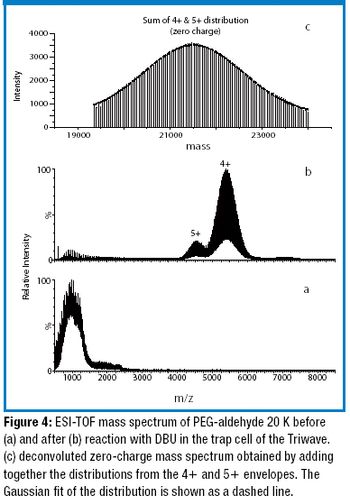
The authors developed a method to accurately measure the average molecular weight of large poly(ethylene glycols) (PEGs) using ion-mobility time-of-flight mass spectrometry coupled with gas-phase ion–molecule reactions.

A roundtable with John Doney, Jiao Yang, Hans Baer, and Elena Draganoiu.

The authors describe the factors affecting reconstitution time of dry powder for injection and classifies them as intrinsic and extrinsic parameters.

Clear labels for substances that can be used as excipients, APIs, or both are critical to end-product use.

Polyethylene glycol (PEG) conjugation is a highly effective technical and commercial strategy to develop macromolecules. The authors explain the benefits and process of PEGylation and how it may be applied to small molecules.

With no economic relief in sight, industry, like all of us, is grappling with high-and new-costs.
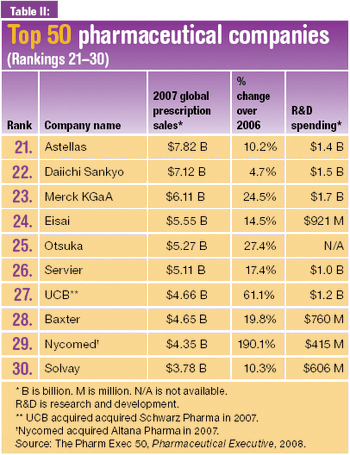
The pharmaceutical majors invest in biologics production capacity as they advance restructuring programs and build their pipelines
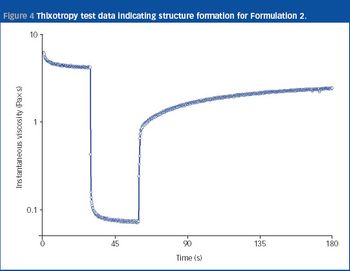
Nasal drug delivery depends on many factors, including the conditions of use by the patient, the drug formulation, and the spray pump and aerosol characteristics. In recent years, the types of drug administered via the nasal route have expanded from locally acting drugs, such as those for allergic rhinitis, to delicate molecules for systemic activity, such as vaccines, proteins and peptides, which can be difficult to administer noninvasively. While the nasal cavity provides a delivery pathway for these large molecules, the rate of mucociliary clearance in the nasal cavity may hinder the extent of absorption. Therefore, formulators must develop mechanisms that improve absorption for high molecular weight compounds.
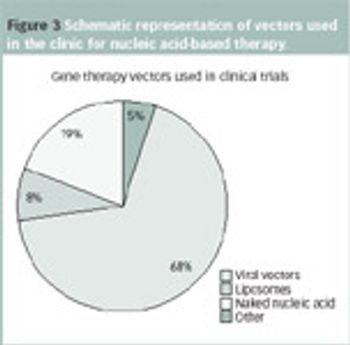
The introduction of biomolecules into cells is a key technology for research in biological sciences.

Biomedical researchers at UT Southwestern Medical Center and nanotechnology scientists at UT Dallas are collaborating on a study that seeks to selectively kill cancer cells using monoclonal antibodies to coat carbon nanotubes that heat up when exposed to near-infrared (NIR) light.

Also, Stiefel Laboratories will acquire Barrier Therapeutics, Danube Pharmaceuticals appoints Brian Levy COO, more...