...an uninformed decision based on commercial requirements alone may, ultimately, have disastrous consequences on the efficacy of the target recombinants.

...an uninformed decision based on commercial requirements alone may, ultimately, have disastrous consequences on the efficacy of the target recombinants.

Also, Zentiva accepts Sanofi's increased takeover bid, Oriel Therapeutics appoints Richard Fuller CEO, more...

Also, Quintiles to expand Singapore operations; Christine A. Poon, chairman of Johnson & Johnson's pharmaceuticals group, to retire; more...

Researchers from the University of California San Diego, UCÂ Santa Barbara, and MIT have developed nanometer-sized hybrid structures carrying anti-cancer drugs and quantum dot imaging agents.

MannKind and Pfizer (New York) agreed that the former company will help patients who need inhaled insulin switch from Pfizer's "Exubera" medicine to MannKind's "Technosphere Insulin" drug.

FDA has issued a Final Rule titled "Amendments to the Current Good Manufacturing Practice Regulations for Finished Pharmaceuticals."

Also, Human Genome Sciences enters pact with Hospira, Zosano Pharma names Gail Schulze chair and CEO, more...

Also, Novartis stops development on "Aurograb," Zealand Pharma appoints David H. Solomon as CEO, more...

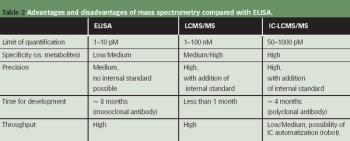
Sterile manufacturing may be the next aspect of pharmaceutical manufacturing to consider in the continuous process paradigm.

Brief pharmaceutical news items for September 2008.

This year, the employment survey will acknowledge the industry's best employers.

With economics and politics in the way, can we defeat the malaria epidemic before it defeats us?

Are hypersanitation trends a result of scaremongering or a lack of faith in medicine?

The author explains the planning, equipment, and facility design requried for manufacturing HPAPIs and specialized requirements for handling these compounds.

We have to make the politicians understand that if we don't take biotech seriously then tourism will be our premier industry in the future.
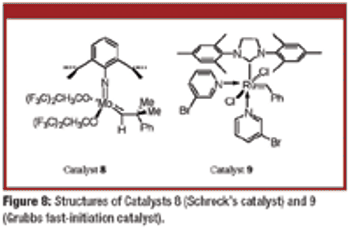
The authors describe the Piers' catalysts and detail latest progress in olefin-metathesis catalyst technology.
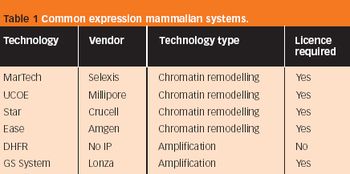
Biotechnological developments have led to an increased number of recombinant proteins or antibodies in drug development that offer high potential in various diseases, such as cancer, growth disturbances and diabetes.
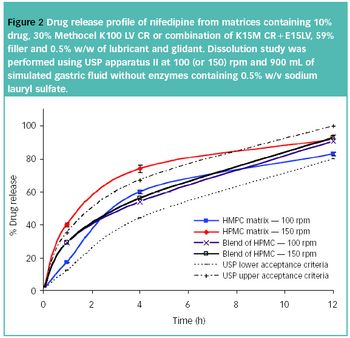
Different chemistries and viscosities of HPMC can be combined to modulate release profile and, in some cases, result in a more robust formulation.
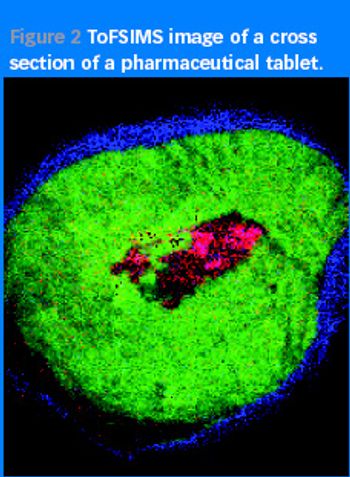
As counterfeiters become more cunning and technologically advanced, spotting their handiwork is increasingly difficult. Can surface analysis techniques be used to outwit them?

The authors describe the critical aspects of an ideal fermentation services provider.

The US Food and Drug Administration will hold a public hearing Oct. 2, 2008, to obtain input regarding over-the-counter (OTC) cough and cold drugs marketed for pediatric use.

The Healthcare Institute of New Jersey (HINJ) released a new research study showing that New Jersey's life-science industry experienced stable growth in 2007.

The deadline for nominations for the Innovations in Pharma Science Awards is here. Check out http://pharmtech.com/Innovations to nominate your company's work in one of five areas by next week

Also, Shire recalls "Daytrana" patch, Chromatide forms Scientific Advisory Board, more...

Also, Tekmira will collaborate with Bristol-Myers Squibb, Helicos Biosciences appoints Steve Lombardi CEO, more...