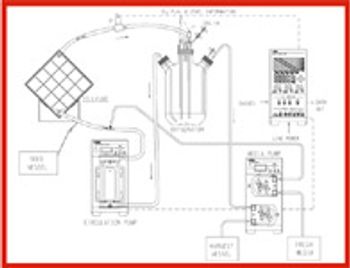
Creating a kinder, gentler manufacturing process that doesn't kill the product is the goal of process developers doing large-scale cell culture for cell therapy.

Creating a kinder, gentler manufacturing process that doesn't kill the product is the goal of process developers doing large-scale cell culture for cell therapy.

Nanoparticle-based systems present many advantages for the delivery of current and emerging biological drugs.

Letting the public inside the drug development process may increase their faith in what we do.

Although the spotlight is now on nanoparticulate delivery for biologicals, other strategies have proven successful.
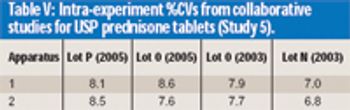
The authors demonstrate that anecdotal reports of prednisone tablet variability are inaccurate.

The good, the bad, and the ugly about direct-to-consumer advertising.

An ambitious survey of characterization techniques presents current information.
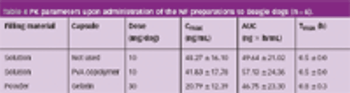
When drugs are encapsulated, electrification (the electrostatic charge of the capsule) may sometimes cause problems, such as capsule adhesion during transportation or dispersion of the capsule content in the filling process.
There are two ways in which 'difficult' samples are usually categorized: either by the problems posed by the physical nature of the post-vivo sample matrix containing the chemical entity to be analysed, or...

Once famous for coal and steel, Wales now aims to make its mark in the life sciences sector. The recent BioWales 2008 (UK) conference demonstrated how much the region has progressed, thanks to the support of the Welsh Assembly government, the EU and the enthusiasm of the Welsh biotech community. The country's bioscience sector, which comprises approximately 200 companies, grew by 18% last year and is now worth £1.3 billion (€1.6 billion) in annual turnover, employing 15–20000 people.

University of Calgary biochemists used computer simulation in a new toxicity study to predict how "buckyballs," carbon-60 soccer ball-shaped nanomolecules, could damage animal cells.

Also, GVK BIO and Wyeth Pharmaceuticals form research agreement, Eli Lilly announces changes to management, more...

Cambridge Consultants is developing a low-cost portable instrument to test respiratory drug-delivery devices.

Also, Pfizer to close Indiana "Exubera" facility, executive appointments at Patheon, more...

Also, Quintiles Transnational to acquire Eidetics, ChemAxon appoints Alex Drijver CEO, more...

Researchers at the University of Southern California's Information Sciences Institute (ISI) demonstrated a way to manufacture miniscule containers called voxels that could potentially deliver precise microdoses or even nanodoses of drugs.

Also, FDA removes OAI status for Watson's Florida facility, executive management changes as GSK, more...

Polyplus-transfection, a company that researches, develops, and commercializes drug-delivery solutions for biomolecules, created a new technology designed to enhance in vivo delivery of small interfering RNAs (siRNAs) when they are associated with a cationic polymer.
Scientists are giving up on a preventive vaccine for AIDS, but there are lessons to be learned.

The authors survey the approved applications of dimethyl sulfoxide USP, PhEur across the healthcare industry and consider the suitability of DMSO from a regulatory and formulation compatibility standpoint.

An authoritative book helps drug developers face one of their toughest problems.

Insulin is one of the world's oldest and most well-known biological drugs, and the need for it is not going to go away as the number of patients diagnosed with diabetes continues to increase. A wealth of clinical evidence shows that good, long-term glucose control in diabetes is key to avoiding complications such as kidney disease, blindness and heart problems.

The current trend within the pharmaceutical industry toward more efficient development, manufacturing, and specification is fueling demand for analytical tools that provide highly relevant information. Effective powder characterization has a valuable role to play.

Paul Sheskey of Dow Chemical provides an update on foam granulation technology.