
However, companies should always be prioritizing prevention, elimination and reduction over recycling and recovery as the most effective ways of making resource efficiency savings.

However, companies should always be prioritizing prevention, elimination and reduction over recycling and recovery as the most effective ways of making resource efficiency savings.

Getting a clear view of business performance can be cumbersome, time-consuming and even nigh-on impossible.

Millipore and Novozymes Form Alliance, Applied Biosystems Names President and CEO, More

Company and People Notes: West to reduce workforce, Eli Lilly's CEO and chairman to retire, more...

Tomorrow, Dec. 14, the US Food and Drug Administration?s Nonprescription Drugs Advisory Committee will discuss the safety and effectiveness of over-the-counter (OTC) cold medications containing phenylephrine.

Company and People Notes: Eisai Acquires MGI Pharma, Ceregene Names CEO, More

Researchers at the University of Kentucky (KY, USA) have located a gene that kills cancer cells while leaving normal cells intact.

Company and People Notes: Boehringer Ingelheim Expands Facility, Regulus Therapeutics Names President and CEO, More

Chi-wan Chen, deputy director of the Office of New Drug Quality Assessment (ONDQA) at the US Food and Drug Administration's Center for Drug Evaluation and Research, shares insight from FDA's pilot program that was designed to allow pharmaceutical companies to submit CMC information demonstrating application of quality by design.
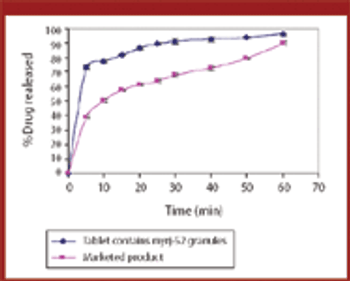
Various manufacturing techniques can improve a drug's solubility, thus increasing its bioavailability. The authors examined whether melt granulation can enhance drug solubility using meloxicam as the drug substance and myrj-52 as the binder.

A surge in capacity in contract microbial and mammalian cell-culture is underway to meet rising production needs for biopharmaceuticals.

Individualized dosing for specific patient needs has been the goal of medical and pharmacotherapy specialists since they first envisioned pharmacogenetic evaluation. With the measurement of individual levels of metabolism, the optimum dose can be calculated for each individual patient.

Nanotechnology offers an unprecedented opportunity in the rational delivery of drugs and vaccines (1, 2–4).
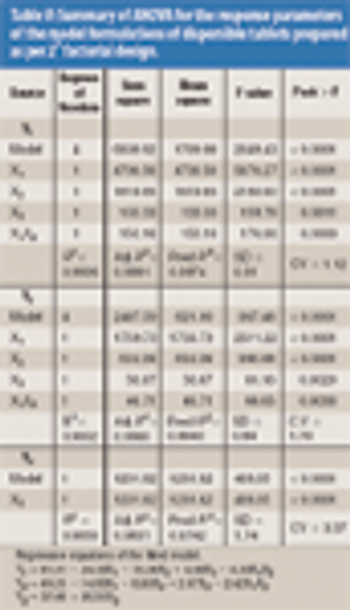
The authors analyzed the effects of complexation as well as the levels of ammonium bicarbonate and crospovidone on tablet wetting time (WT), disintegration time (DT), and percent dissolution efficiency at 60 min (%DE60).

"May you live in interesting times," goes the allegedly ancient Chinese curse. Well, these have been "interesting times" indeed for those working in the pharmaceutical industry.
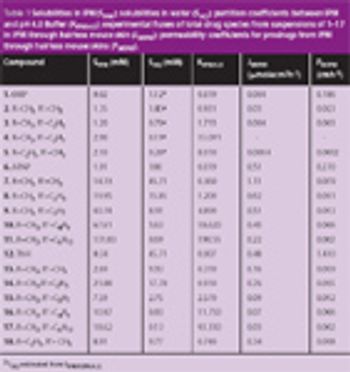
The permeation of drugs through the skin is compromised by the presence of polar functional groups such as thiols, alcohols, phenols, imides or amides. By transiently masking these polar functional groups as prodrugs the permeability of drugs containing these functional groups through the skin can be improved.

Incorporating quality and economy into downstream purification processes can expedite first-in-human clinical trials, product licensure and technology transfer. Experience in the chromatographic purification of biopharmaceuticals enables the use of downstream processing heuristics to produce target molecules in cost-effective processes suitable for regulatory scrutiny.
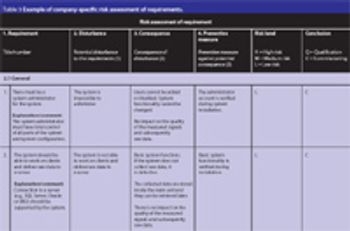
It is becoming evident that quality risk management within regulated, life sciences environments is a valuable component of an effective quality management system (QMS). A QMS provides a proactive and systematic means to identify, analyse, evaluate and control potential process and product quality issues during development, manufacturing, distribution and marketing throughout the entire product life cycle.

A well-devised QPP, which has been agreed on and signed by both parties, saves time and makes it easier to complete activities such as design, installations and tests.
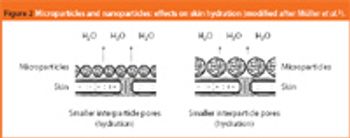
Lipid-based drug delivery systems - such as liposomes, micro-and nanoemulsions, self-emulsified drug delivery systems, and solid lipid micro-and nanoparticles - are becoming more popular because lipid materials are easily characterized, contain a high range of well-defined/tolerated surfactant molecules and can be developed for several administration routes.

Never has greater pressure been applied to pharmaceutical manufacturers. Shelf space competition for branded drugs has reached aggressive proportions and now even prescription drugs vie for pricing and delivery. Against this is a backdrop of ever-increasing downward price pressures, and a spectrum of progressively more stringent legislative and quality requirements. Finally, regional markets now demand different tamper evidence technology, anticounterfeiting measures and safeguards against interference by biological terrorists. Much of which points to the need for innovation in packaging - not just in terms of pack styles and sizes, but also cost.

Company and People Notes: ISP to raise prices, Patheon appoints CEO, more.

Company and People Notes: Cambridge Major Labs acquires ChemShop; Helix BioPharma restructures senior positions, more.

Company and People Notes: BASF raises prices on excipients; Verus Pharmaceuticals appoints president and CEO; more.

A company has raised ?700000 to assist in the development of treatments for antibiotic resistant bacteria.