
Scientists at The Wistar Institute are taking further steps into understanding a gene regulator that can lead them closer to developing new cancer therapies.


Scientists at The Wistar Institute are taking further steps into understanding a gene regulator that can lead them closer to developing new cancer therapies.

Chesapeake to relocate and expand, AVI BioPharma appoints CEO, More...

Amira and GSK Form Agreement, Xenome Appoints Ian Nisbet CEO, More...

Contract manufacturers expand capabilities in aseptic processing, clinical-trial materials supply, and cytotoxic manufacturing.

The rise in overseas manufacturing undermines FDA oversight of drug quality.

PharmTech's polls feature user feedback on issues facing the pharmaceutical industry.

Brief pharmaceutical news items for February 2008.

A news roundup for February 2008.

Can an overload of patent applications lead to the US' demise as a scientific leader?

An updated book summarizes recent research for formulators and drug-delivery specialists.

A wave of pharmaceutical expansions is expected in Europe this year, surprisingly by Indian companies.

Text sirtuins are the new kinases, according to a presentation given last month at the JP Healthcare Investors Conference in San Francisco by Sirtis CEO Christoph Westphal.

Codexis Opens Budapest Lab, Allos Names Bruce K. Bennett VP of Manufacturing, More...

We are currently experiencing a problem with one of our tablet lines. While the tablets appear white immediately after manufacture, after a time many of the tablets begin to take on a yellowish appearance. Could this be an issue that surface analysis could help resolve?

Actavis Acquires Site From Pfizer, Cardiokine Names President and CEO, More...

Novo Nordisk Drops Inhaled Insulin Product, Neurocrine Appoints CEO and President, More...

Crucell and Sanofi Pasteur Sign Agreement, WuXi Pharma Names COO, More...

The National Nanotechnology Initiative's new strategic plan affirms the federal government's support for research and development in nanotechnology, including for medical and healthcare applications.

Merck Sells Facility to Cherokee Pharmaceuticals, Inflazyme Announces Senior Management Resignations, More...
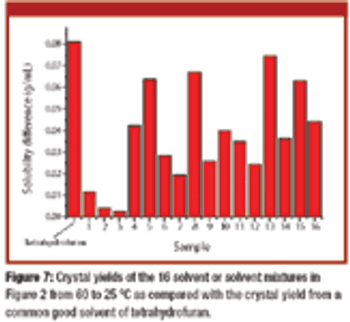
The authors propose extending initial solvent screening for a single-solvent system to the cocktail solvent screening of binary and ternary solvent mixtures.

FDA’s May 1999 container-closure guidance has accelerated the requirements for extractable and leachable testing of container-closure packaging components. Since that date, additional recommendations have been made by industry groups to clarify how these issues should be addressed.
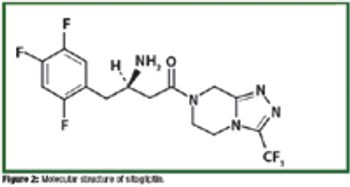
Researchers and process chemists share approaches in synthesis of active ingredients and pharmaceutical intermediates.